Tibialization of the distal fibula with preservation of the epiphyseal plate
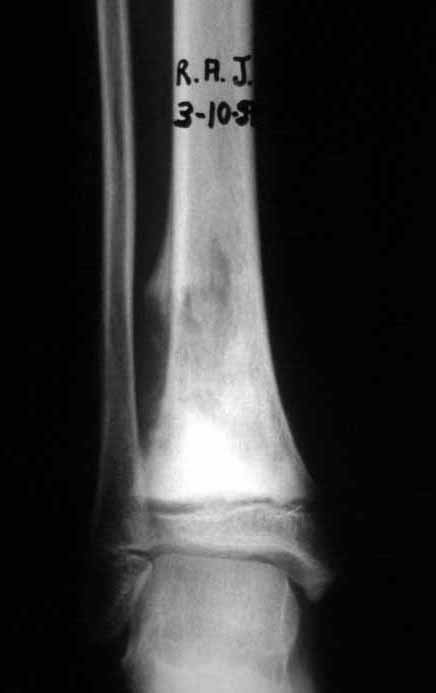
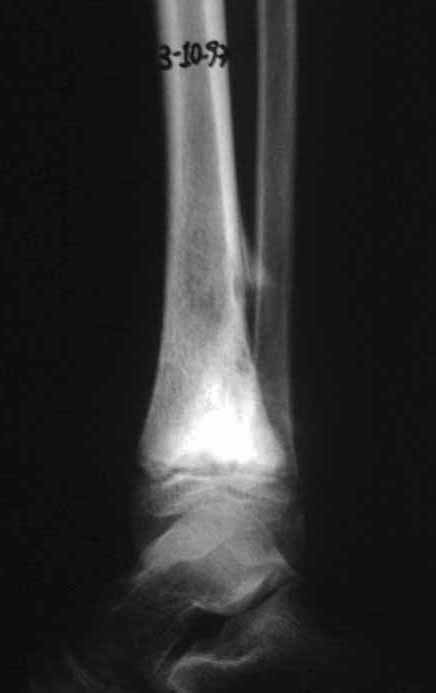
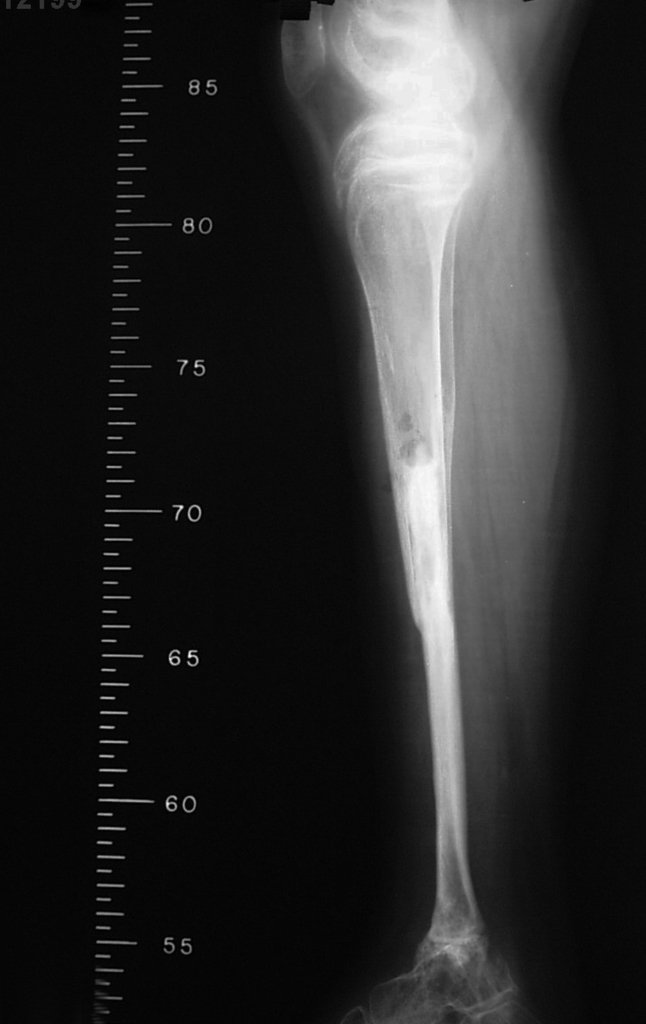
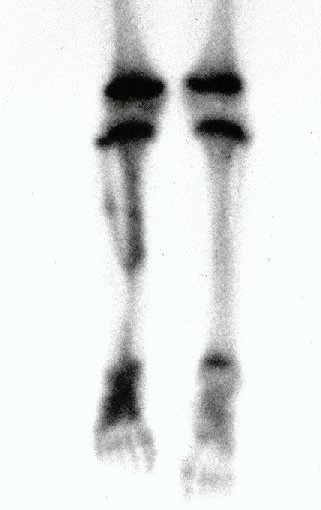
Reconstruction was performed by arthrodesing the ipsilateral distal fibular epiphysis with the talus, preserving the fibular epiphyseal plate. Preliminary postoperative evaluation using axial slice scintigraphy demonstrated signs of capture of the fibula throughout the transposed extension and at the level of the projection of the distal fibular growth plate. It is not yet possible to distinguish the hypercapture of the physeal plate from the reparative process of arthrodesis at the level of the talus. Radiographic controls from September/98, nine months after surgery, show complete integration of the transposed fibula, both proximally and distally. Thickening of the fibula is already evident and the fibular growth plate can be easily distinguished.
INTRODUCTION: Osteosarcoma of the tibia in a child
Osteosarcoma is the most common primary malignant bone tumor between the first and second decades of life(21). It generally affects the metaphyses of long bones, with the most common locations being the distal third of the femur and the proximal third of the tibia. Location in the distal third of the tibia represents approximately 3% of cases.
With the evolution of chemotherapy treatment, there was new encouragement in the approach to this condition, as it provided an increase in the average survival rate(2,7,19,21,22). This fact led to better improvements in the surgical techniques used until then. Malignant neoplasms previously treated with radical surgery, currently, when they respond favorably to neo-adjuvant chemotherapy, are approached with the aim of preserving the involved limb, with or without a biological solution(1,2,4,8,13,14,22). This concept has expanded, raising the expectations of the surgeon who seeks to combine the preservation of the affected body segment with the maintenance of maximum function(4,8,13).
CASE REPORT
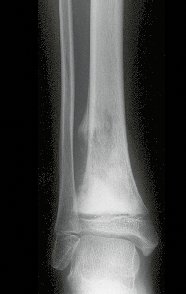
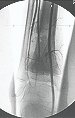
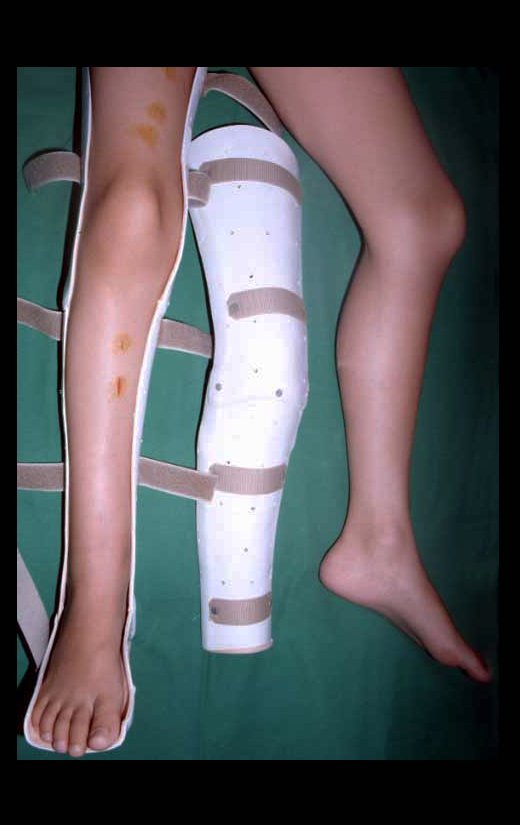
Female child, nine years and three months old, with a history of direct trauma to the right ankle two months ago, evolving with pain and local edema. She sought medical treatment and was diagnosed with a contusion. Plaster immobilization was performed for six days. Fifteen days later, she noticed an increase in volume in her ankle, which was painful and hardened, and sought our service. The radiograph revealed a radiolucent lesion, centrally located, in the distal third of the tibia, with imprecise radiographic limits and a thin laminar periosteal reaction (fig. 1). Bone scintigraphy showed intense uptake only at the site and nuclear magnetic resonance (fig. 2) showed intense involvement of the meta-epiphyseal region, with evident involvement of the tibial epiphyseal plate. Laboratory tests demonstrated changes in bone metabolism, with very high alkaline phosphatase and serum calcium. We performed a needle biopsy, and the diagnosis of chondroblastic osteosarcoma was confirmed. Neo-adjuvant chemotherapy treatment began, with three cycles of chemotherapy. As part of the pre-operative surgical planning, we performed arteriography (fig. 3) to visualize the emergence of the nutrient artery of the fibula, a time that we considered important to identify the safe site for the osteotomy and its transposition. A polyethylene cruropodal orthosis was made prior to surgery, aiming for adequate immobilization, providing better support for the limb in the postoperative period (fig. 4). After neo-adjuvant chemotherapy, she underwent surgical treatment.
DESCRIPTION OF OPERATIVE TECHNIQUE
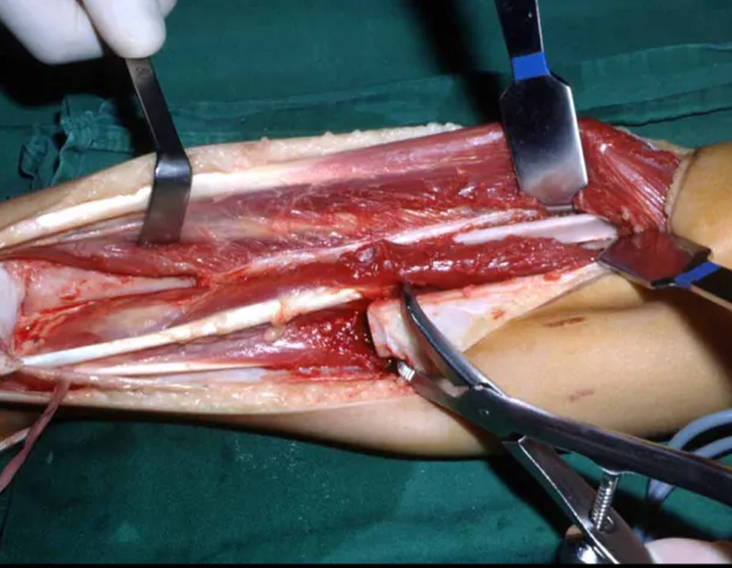
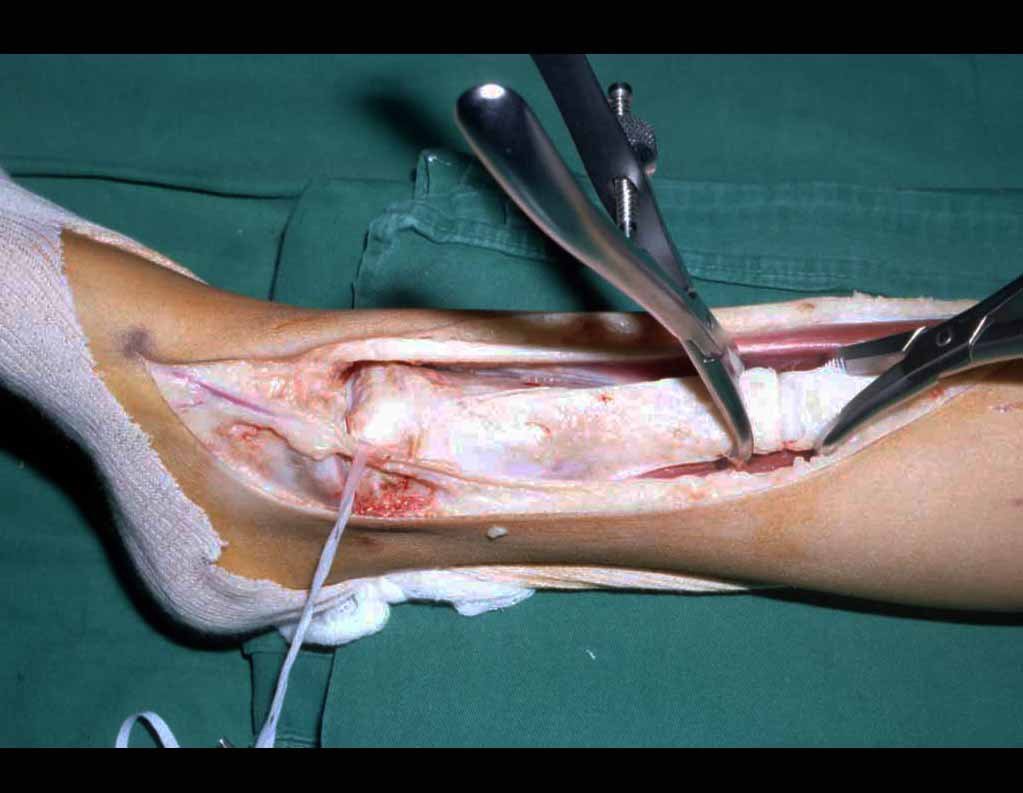
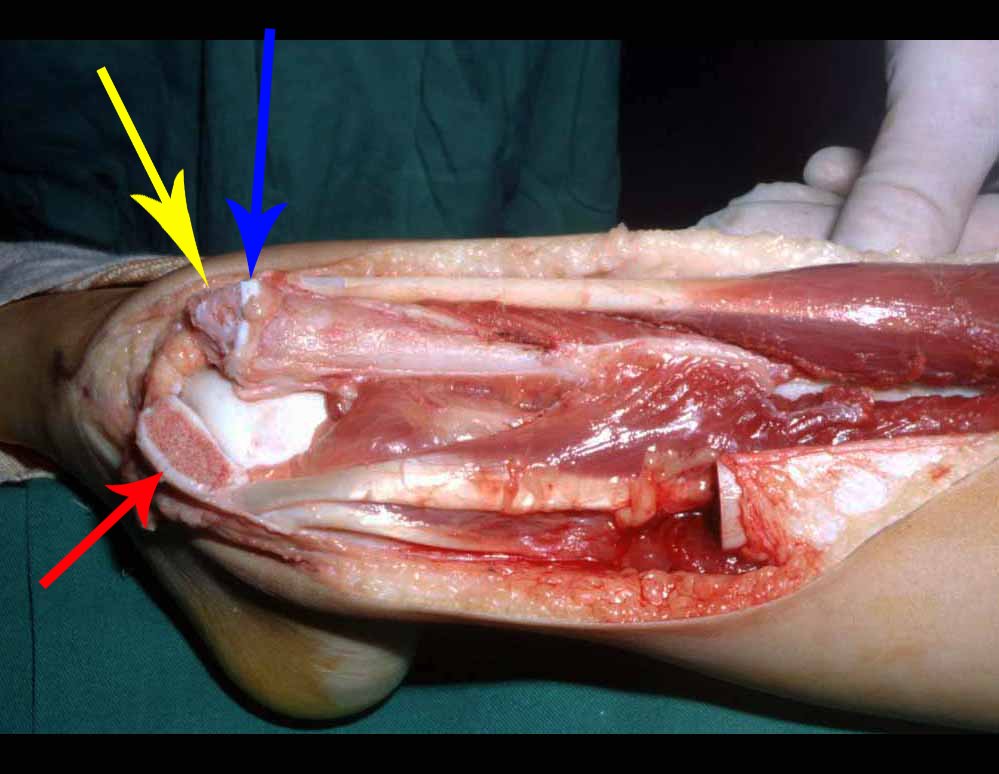
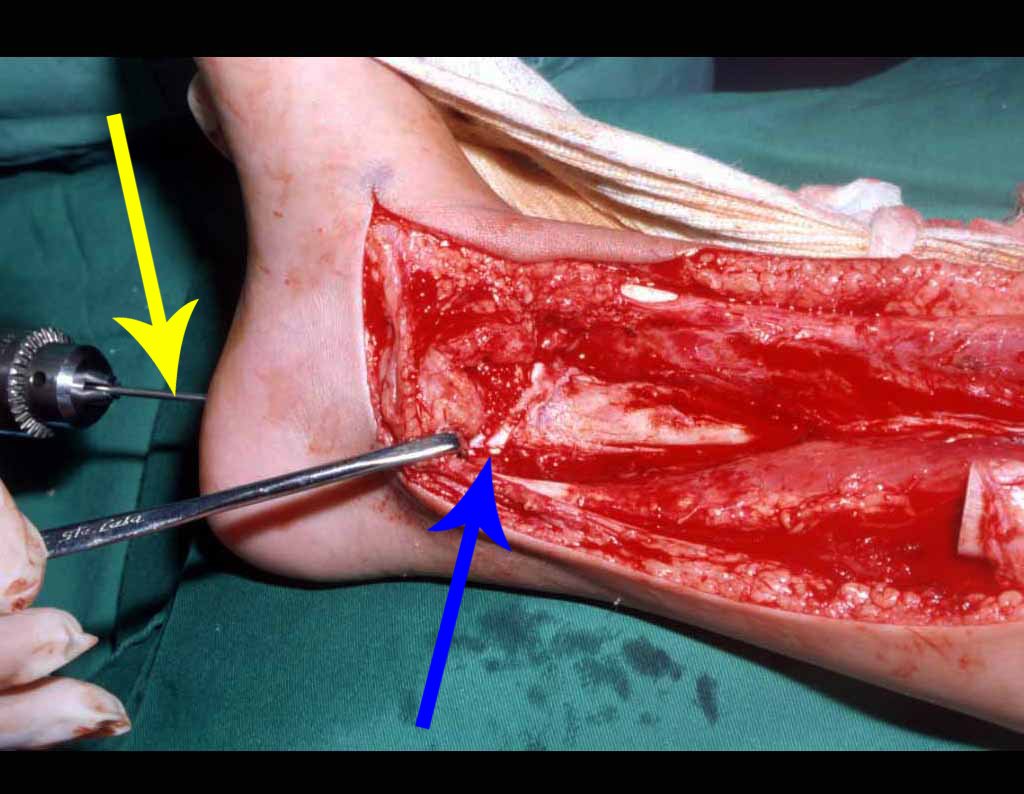
The surgery is performed with a medial convex arcuate incision starting at the level of the head of the fibula, passing through the anterior surface of the leg, up to the end of the lateral malleolus. The lesion is resected with a macroscopic oncological margin in the soft tissues and a 3.0 cm bone margin (fig. 5). After resection of the tumor, using the interosseous membrane as a guide, we approached the site of the osteotomy of the proximal fibula, above the emergence of the nutrient artery, confirmed by a previous arteriographic study (fig. 6). In this case, as the resection of the tibial segment was smaller, we opened a slit on the lateral surface of the proximal segment of the tibia, approximately 3.5 cm long and wide enough to enable its interlocking, with minimal deperiostization. from the proximal end of the transposed segment, and without harming the nutrition provided by the nutrient artery. Next, we remove the cartilage from the fibular epiphysis and carve a hole in the dome (fig. 7) of the talus, allowing this distal fibular epiphysis to fit. We continued with the careful passage of a 2.5mm diameter wire through the medullary canal of the fibula, crossing the physeal plate. This thread continues through the epiphysis and is passed through the talus and calcaneus until it appears on the skin (fig. 8).
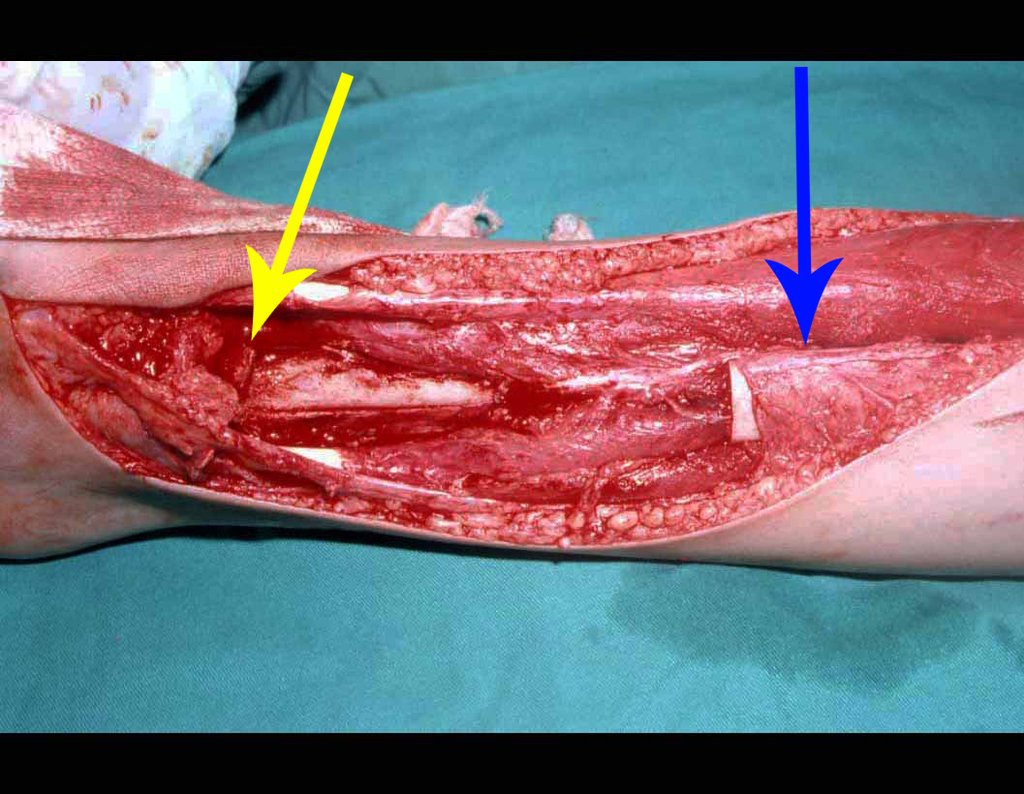
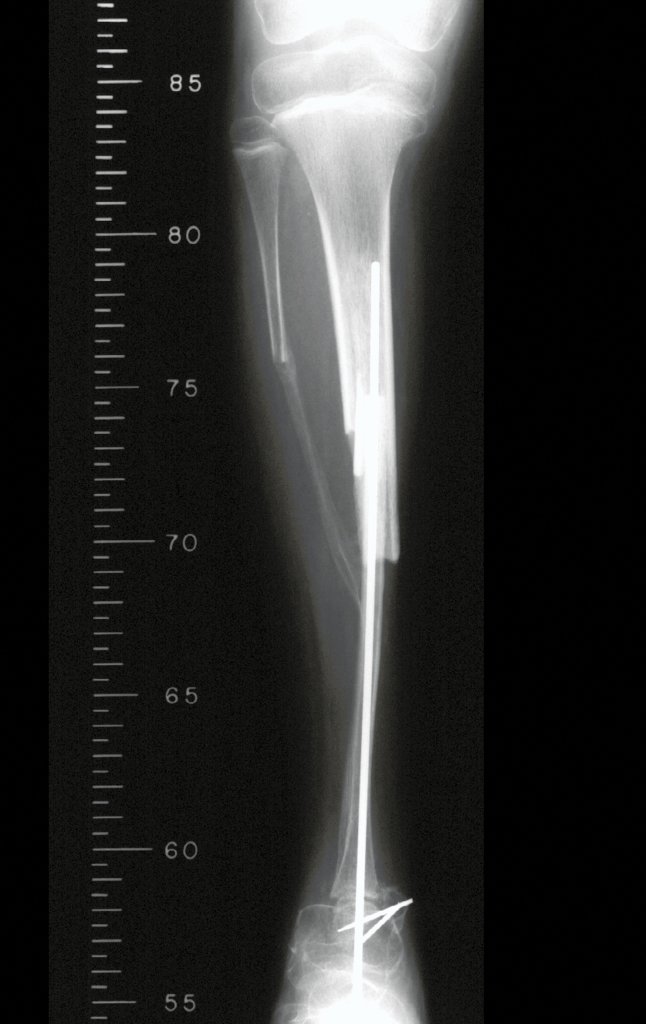
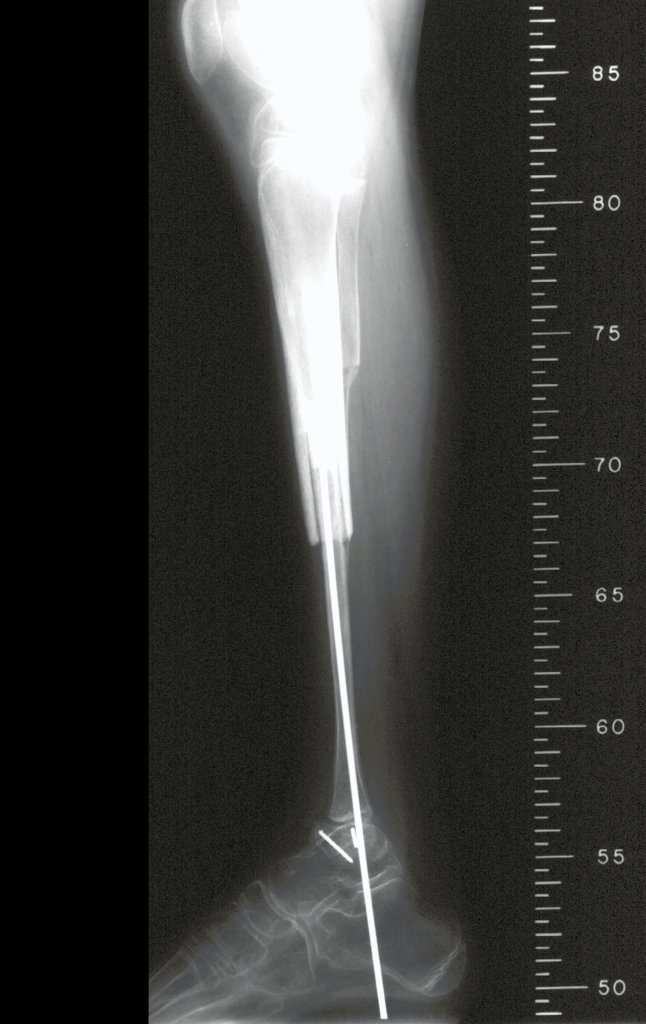
In the immediate postoperative period, the limb was kept in immobilization with the previously made cruropodal device. Six weeks after surgery, we performed bone scintigraphy with axial sections, confirming good vascularization of the graft (fig. 11). In the metaphyseal region of the transplanted fibula, the increase in hyperuptake may be due to the vascularization of the physeal plate itself and also to the reparative process at the site of the talofibular arthrodesis. Radiographic controls from September/98, nine months after surgery, show complete integration of the transposed fibula, both proximally and distally. The thickening of the fibula is already evident and its growth plate can be easily distinguished (fig. 12).
DISCUSSION
Advances in polychemotherapy in the treatment of osteosarcoma have brought new perspectives regarding the prognosis and approach of affected patients. Controlling the disease through chemotherapy made it possible to preserve limbs, allowing new possibilities and the most varied solutions to be proposed(4,8,13). One of the solutions was the replacement of the affected segment with non-conventional internal prostheses. However, in young children, there are basically two major problems: patients continue to grow and prostheses become insufficient, making amputation necessary in some cases, often years after the start of treatment(5 .6); Furthermore, prostheses suffer excessive wear and young patients have to undergo early revisions. Prosthetics in children have very limited indications(6). With the considerable increase in survival, it became necessary for the orthopedic surgeon to look for long-lasting limb-saving surgical solutions. The use of a homologous graft to fill the bone gap is an option. However, in addition to the greater difficulty in integrating the graft, the greater number of complications, even exceeding those of endoprostheses, it also requires a bone bank, which is not always possible in our reality(6). The use of autologous grafts is sometimes limited when there is a need to replace large resections. Vascularized bone grafting has been used more frequently and presents good results. When replacing segments of the tibia, the option has been to use the ipsilateral or contralateral vascularized fibula, and several techniques have been proposed(3,5,9-12,15-18,23-25). Some of these techniques are performed in two operative stages, which increases morbidity. Microsurgical techniques are also used, but they require a specialized team, with prolonged surgical time. The technique presented here is quick, easy to perform, performed in a single surgical procedure and does not require a microsurgical technique. In an attempt to preserve the length of the limb, we transposed a segment of the fibula with the distal physis, hoping that it will remain active. We cannot yet say, due to the short follow-up period, that preservation of the physis in the fibular transposition technique will lead to bone growth, nor how this growth will occur.
PRELIMINARY CONCLUSIONS
The biological solution in the treatment of osteosarcoma is an increasingly common reality in our country and should always be considered. We believe that the presence of bone hyperuptake at the level of the projection of the physeal plate of the distal fibula in bone mapping exams may be evidence that it is viable, although it is impossible to distinguish how much of this process is due to bone reaction at the level of fixation of the epiphysis of the fibula in the body of the talus. Considering the short follow-up period and the fact that this is a single case, it is not possible to definitively evaluate the treatment method used. What we can say with satisfaction, at the moment, is that the radiographic controls from September/98, nine months after surgery, show complete integration of the transposed fibula, both proximally and distally. Thickening of the fibula is already evident and the fibular growth plate can be easily distinguished. We believe that growth will occur and we hope that there will be adaptation of this physeal plate, so that it grows with the speed of the tibia, as we know that the speed of growth is also influenced by the location in which it is located.
REFERENCES:
1. Bacci, G. et al: Primary chemotherapy and delayed surgery for non-metastatic telangiectasic osteosarcoma of the extremities: results in 28 patients. Eur J Cancer 30A: 620-626, 1994.
2. Campanacci, M.: “Classic osteosarcoma”, in Campanacci, M. et al: Bone and soft tissue tumors, Bologna, Aulo Gaggi Ed., 1990. p. 455-480.
3. Campbell, WC: Transference of the fibula as an adjunct to free bone graft tibial deficiency: report of three cases. J Orthop Surg 1:625, 1919.
4. Carter, SR, Grimer, RJ & Sneath, RS: A review of 13-year experience of osteosarcoma. Clin Orthop 270: 45-51, 1991.
5. Chacha, PB: Vascular pedicle graft of the ipsilateral fibula for non-union of the tibia with a large defect. J Bone Joint Surg [Br] 63: 244- 253, 1981.
6. David, A. et al: Osteosarcoma: review of 39 cases. Rev Bras Ortop 33: 45-48, 1998.
7. Davis, AM, Bell, RS & Goodwin, PJ: Prognostic factors in osteosarcoma: a critical review. J Clin Oncol 12: 423-431, 1994.
8. Dubousset, J., Missenard, G. & Kalifa, C.: Management of osteogenic sarcoma in children and adolescents. Clin Orthop 270: 52-59, 1991.
9. Girdlestone, GR & Foley, WB: Extensive loss of tibial diaphysis. Ti-bio-fibular grafting. Br J Surg 20: 467-471, 1933.
10. Hahn, E.: Eine methode, pseudoarthrosen der tibia mit grossem knochen defect zur heitung zubringen. Zentralbl Chir 11: 337-341, 1884.
11. Huntington, TW: Case of bone transfer. Ann Surg 41: 249-251, 1905.
12. Jones, KG & Barnett, HC: Cancellous bone grafting for non-union of the tibia through the posterolateral approach. J Bone Joint Surg [Am] 37: 1250-1260, 1955.
13. Lane et al.: Osteogenic sarcoma. Clin Orthop 204: 93-110, 1986.
14. Marwin, MR: Amputation for osteosarcoma. Cancer Bull 42: 337-343, 1990.
15. McCarrol, HR: The surgical management of ununited fractures of the tibia. JAMA 175: 578-583, 1961.
16. McMaster, PE & Hohl, M.: Tibiofibular cross-peg grafting. J Bone Joint Surg [Am] 47: 1146-1158, 1965.
17. Meyerding, HW: Tibial defects with non-union created by the transfer of the fibula and tibiofibular fusion. Am J Surg 52:397-404, 1941.
18. Milch, H.: Synostosis operation of persistent non-union of the tibia. A case report. J Bone Joint Surg [Am] 21: 409-420, 1939.
19. Petrilli, S. et al: IIB osteosarcoma. Current management, local control and survival statistics – São Paulo, Brazil. Clin Orthop 270: 60-66, 1991.
20. Picci et al: Relationship of chemotherapy-induced necrosis and surgical margins to local recurrence in osteosarcoma. J Clin Oncol 12: 2699-2705, 1994.
21. Simon, AM & Springfield, D.: Surgery for bone and soft tissue tumors, Baltimore, Lippincott-Raven, 1998. p. 266.
22. Spanier, SS, Shuster, JJ & Griend, RAV: The effect of local extent of the tumor on prognosis in osteosarcoma. J Bone Joint Surg [Am] 72: 643-653, 1990.
23. Stone, JS: Partial loss of the tibial replaced by transfer of the fibula, with maintenance of both malleoli of the ankle. Ann Surg 46: 628-632, 1907.
24. Taylor, GI & Millar, GDH: The free vascularized bone graft, a clinical extension of microvascular techniques. Plast Reconstr Surg 55: 533-544, 1975.
25. Wilson, PO: A simple method of two-stage transplantation of the fibula for use in cases of complicated and congenital pseudoarthrosis of the tibia. J Bone Joint Surg [Am] 23: 639-675, 1941.
Click here to download the PDF
Click here to see the complete technique in more detail.
Read too:
Use of extensible internal device in the femur of young dogs .
Author: Prof. Dr. Pedro Péricles Ribeiro Baptista
Orthopedic Oncosurgery at the Dr. Arnaldo Vieira de Carvalho Cancer Institute
Office : Rua General Jardim, 846 – Cj 41 – Cep: 01223-010 Higienópolis São Paulo – SP
Phone: +55 11 3231-4638 Cell:+55 11 99863-5577 Email: drpprb@gmail.com