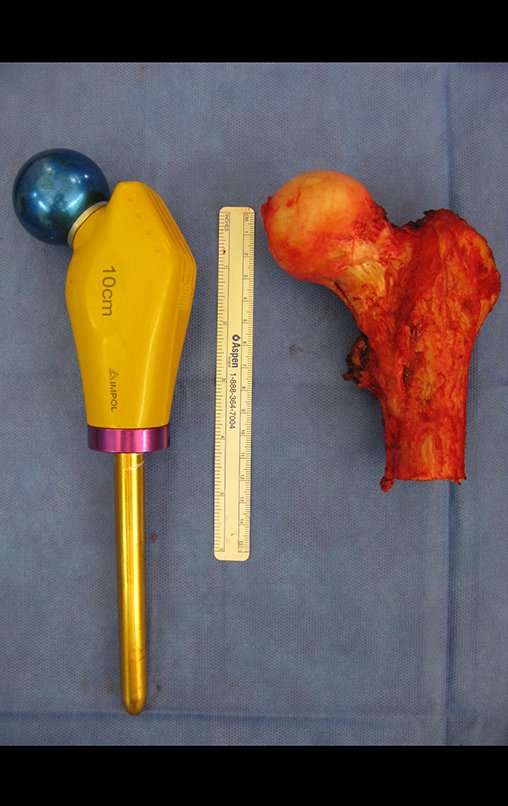
Radiotherapy – Technique for resection of bone metastasis from a kidney tumor in the femur – Reconstruction with polyethylene endoprosthesis
In the orthopedic evaluation at this time, the patient did not present significant symptoms.
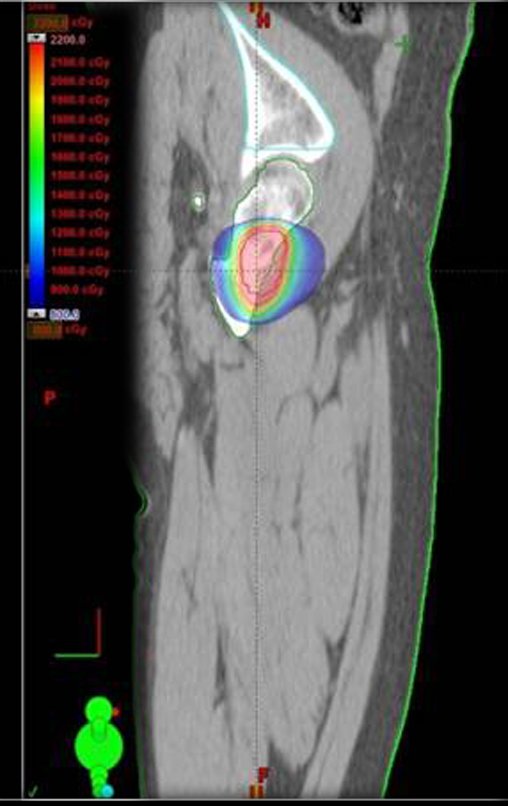
We considered the short period of radioablation and chemotherapy treatment, as well as the risk of fracture.
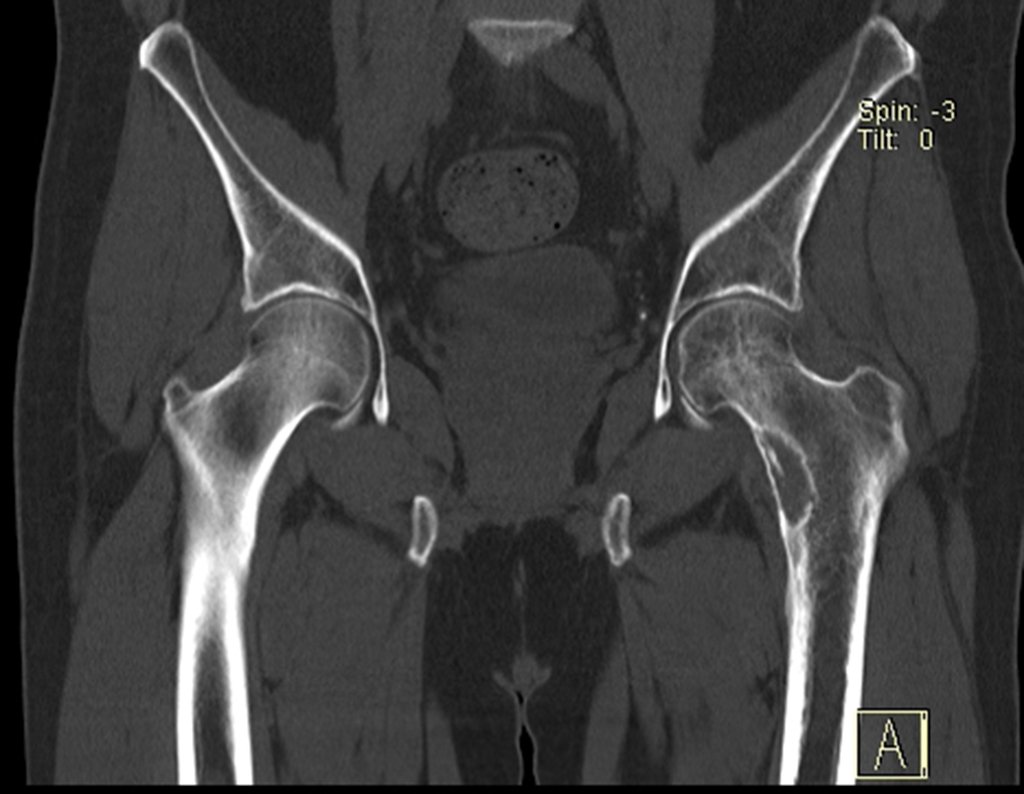
The medullary irrigation of the femoral neck in adults is retrograde, from the metaphysis to the epiphysis. The main irrigation of the epiphysis is through the posterior circumflex artery, which may have been the route of metastatic dissemination and may even be compromised. To make matters more difficult, the femoral neck has a very weak periosteum, with little capacity for bone regeneration, which is the cause of many failures in bone consolidation when fractures occur in this region.
Together with the patient and family, we decided to wait, trying to give more time and opportunity for bone repair. We chose to reevaluate in July, with new imaging tests, paying attention to the symptoms.
Postponing surgery is a difficult decision. The expectation and anxiety is shared and experienced by everyone.
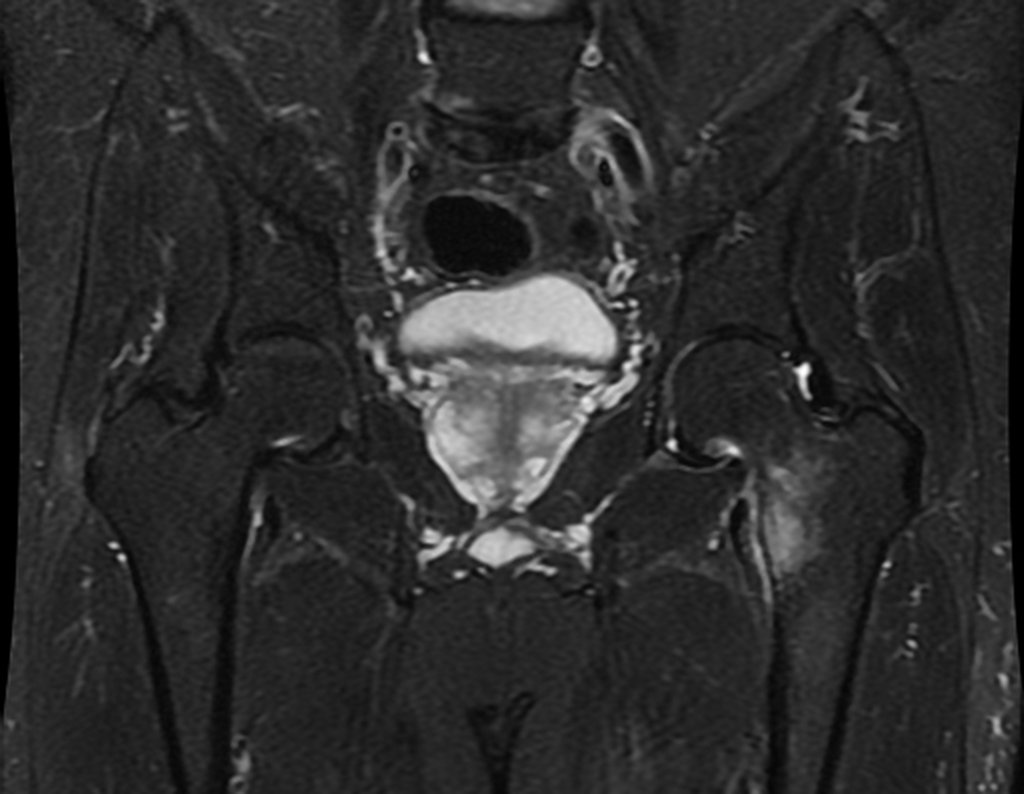
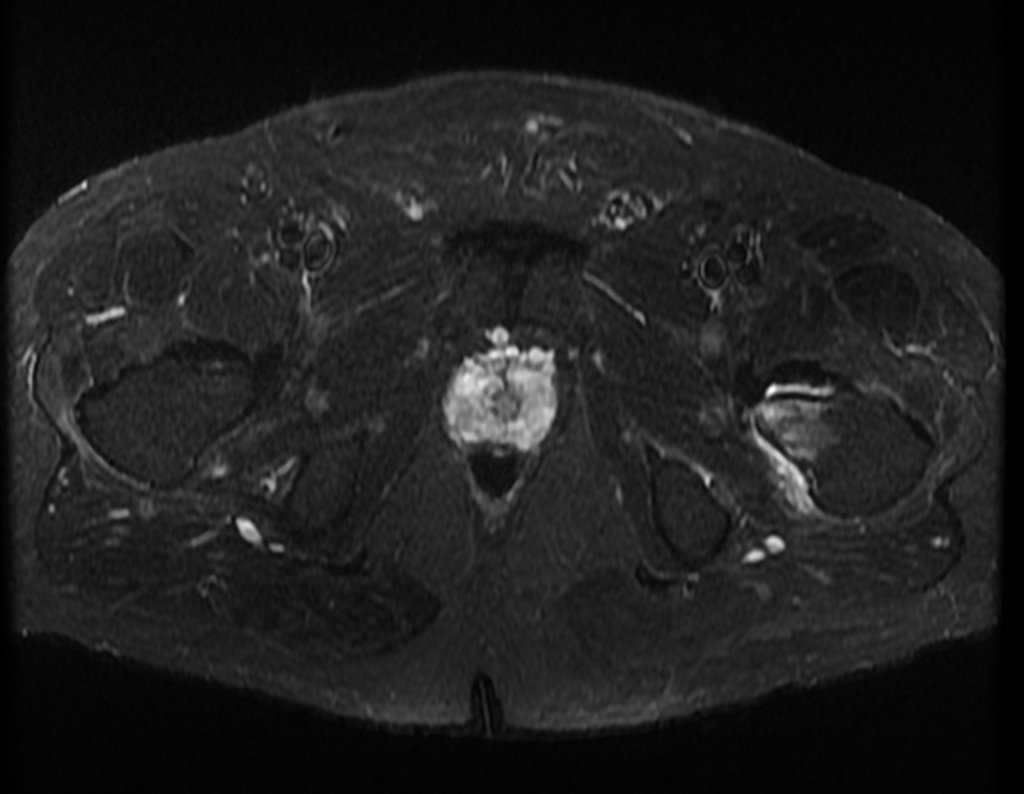
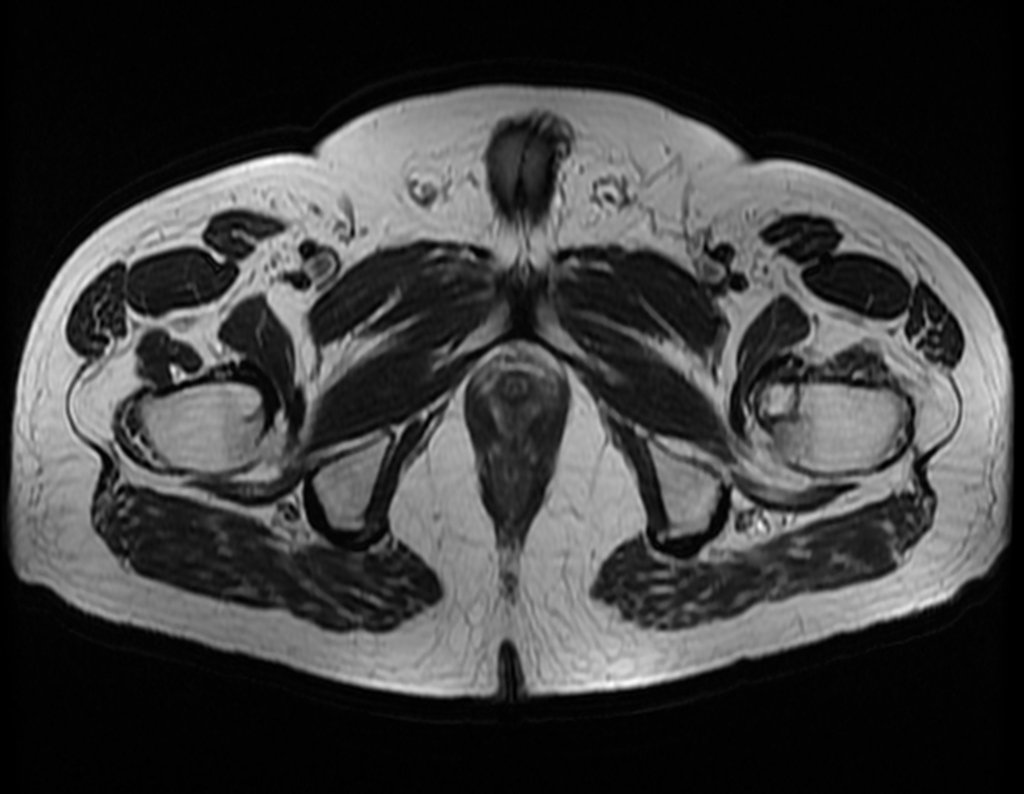
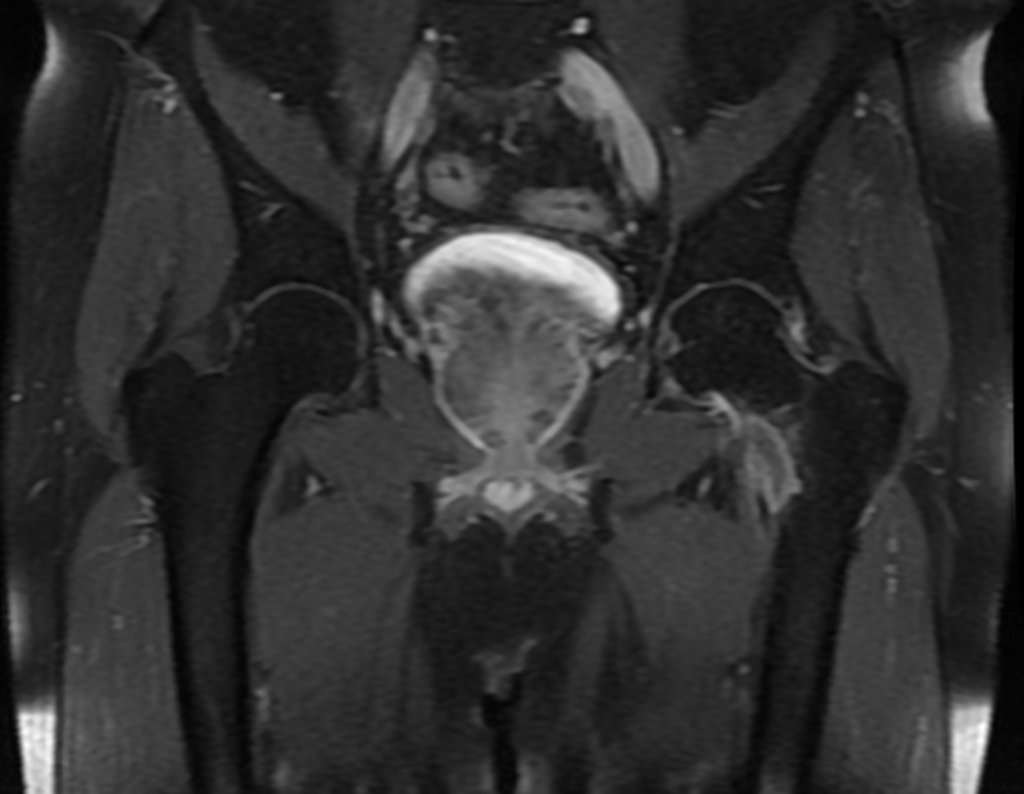
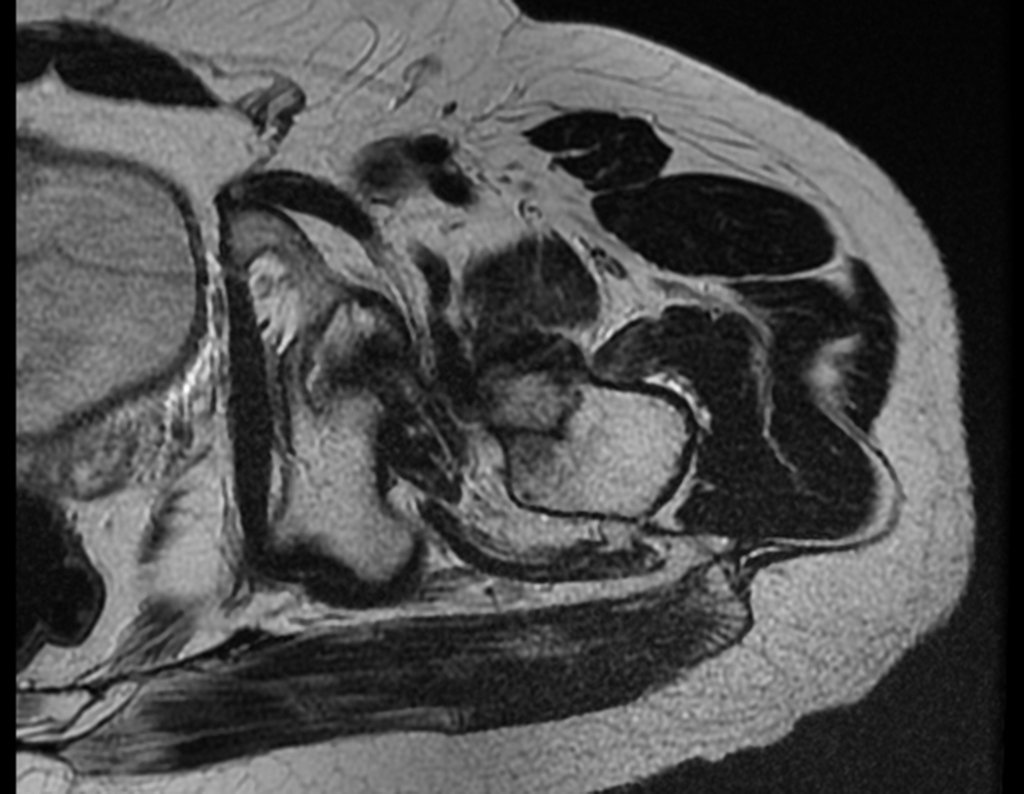
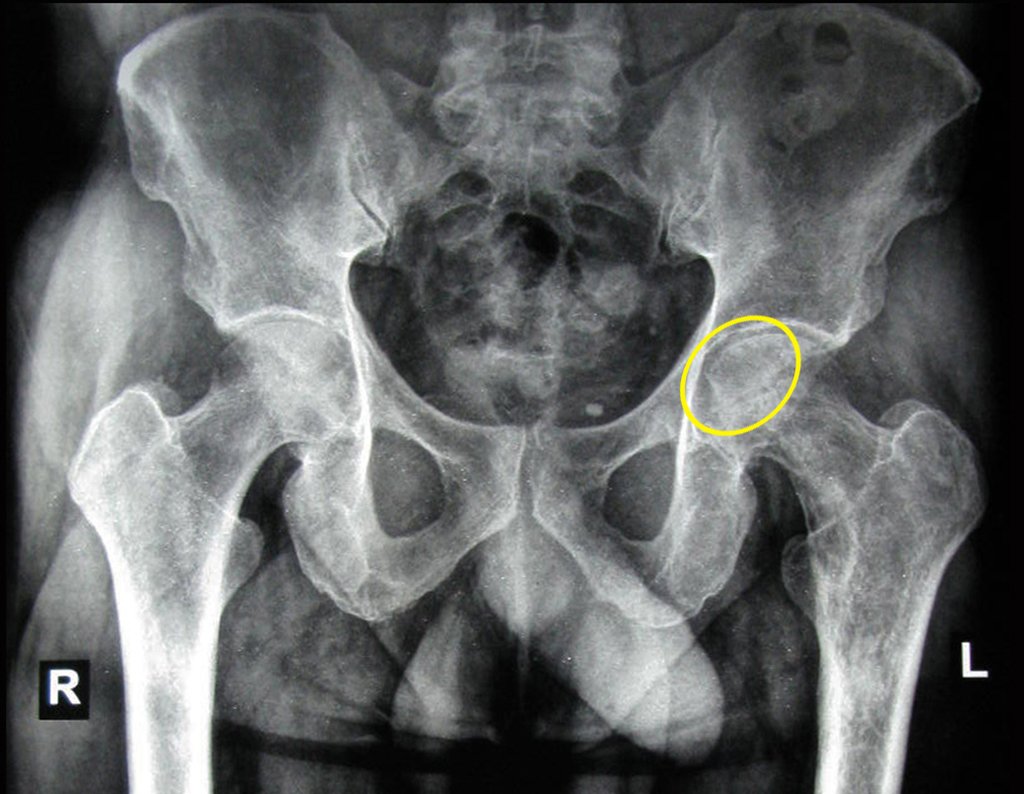
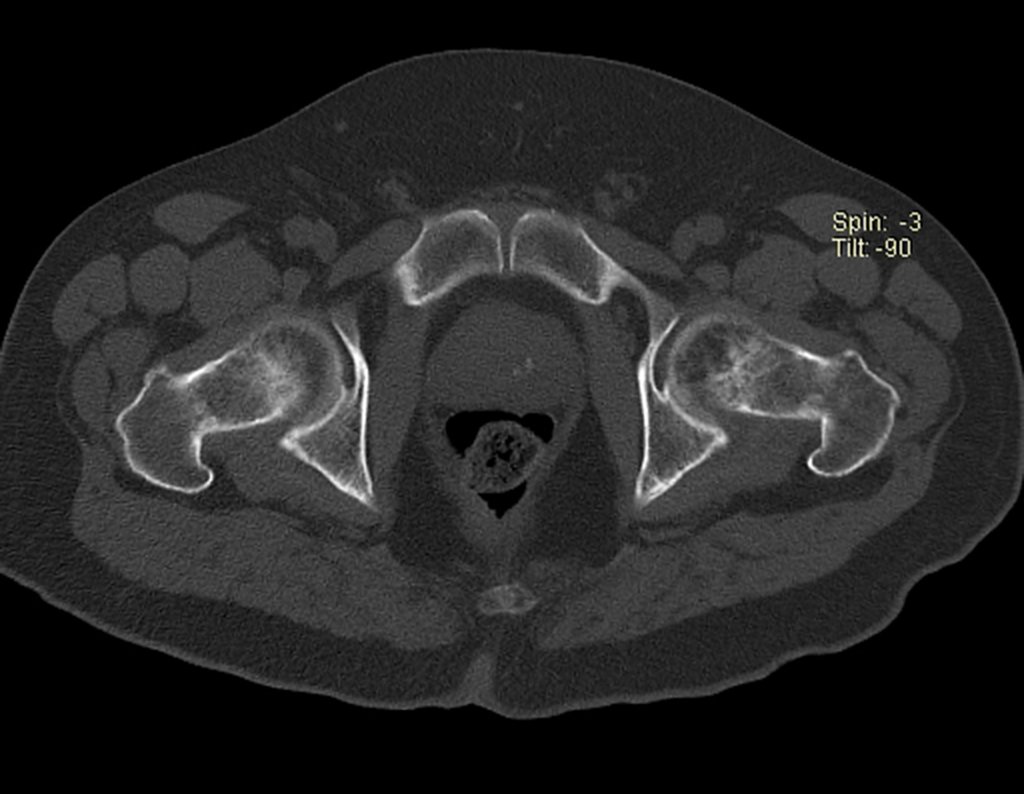
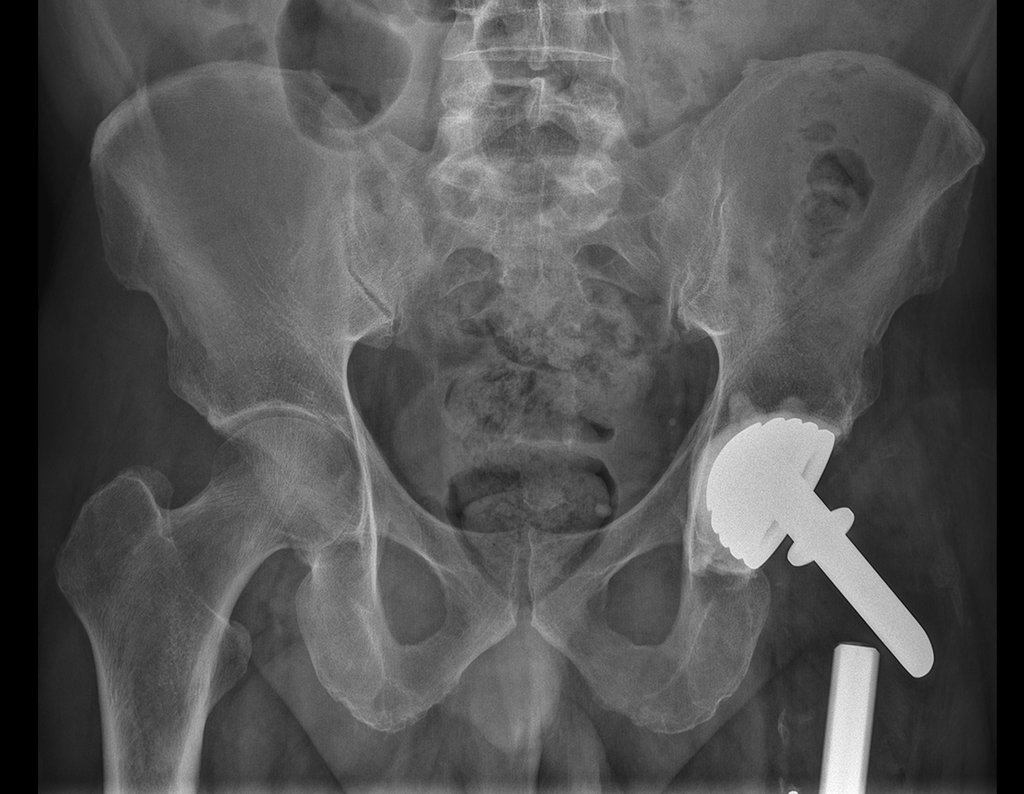
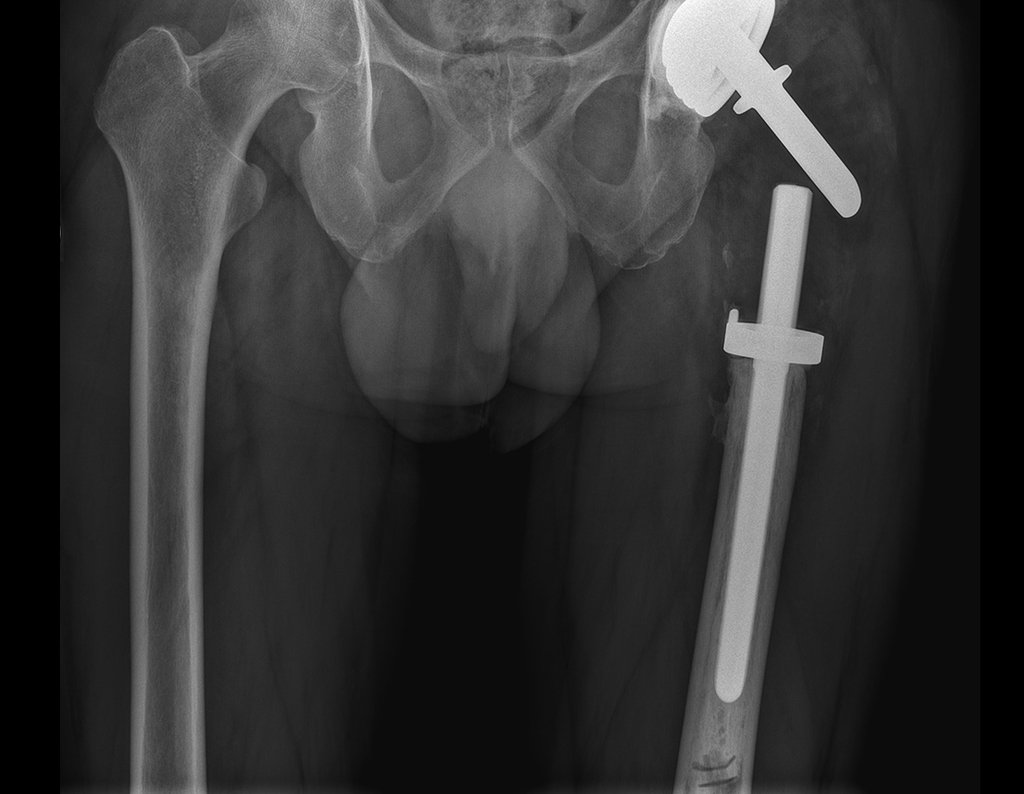
The patient returns on July 22, 2015, complaining of pain when moving from sitting to standing, pain when rotating the hip and limping. The imaging exams, from the MRI on July 18, 2015, are analyzed in figures 39 to 59.
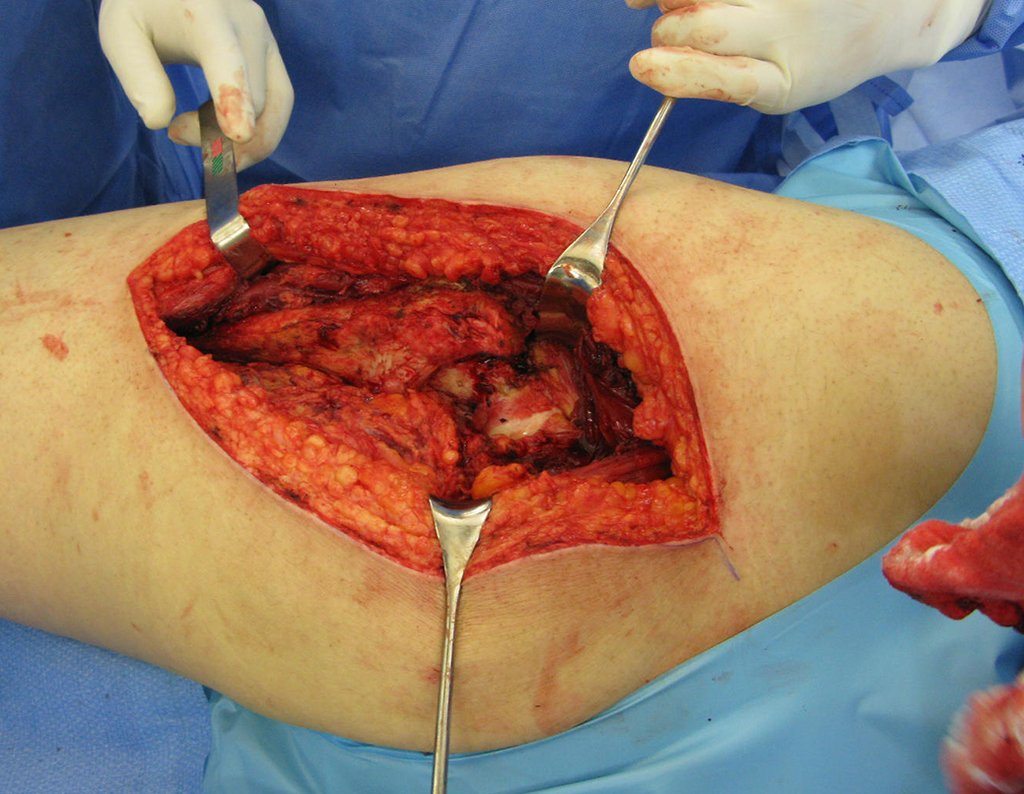
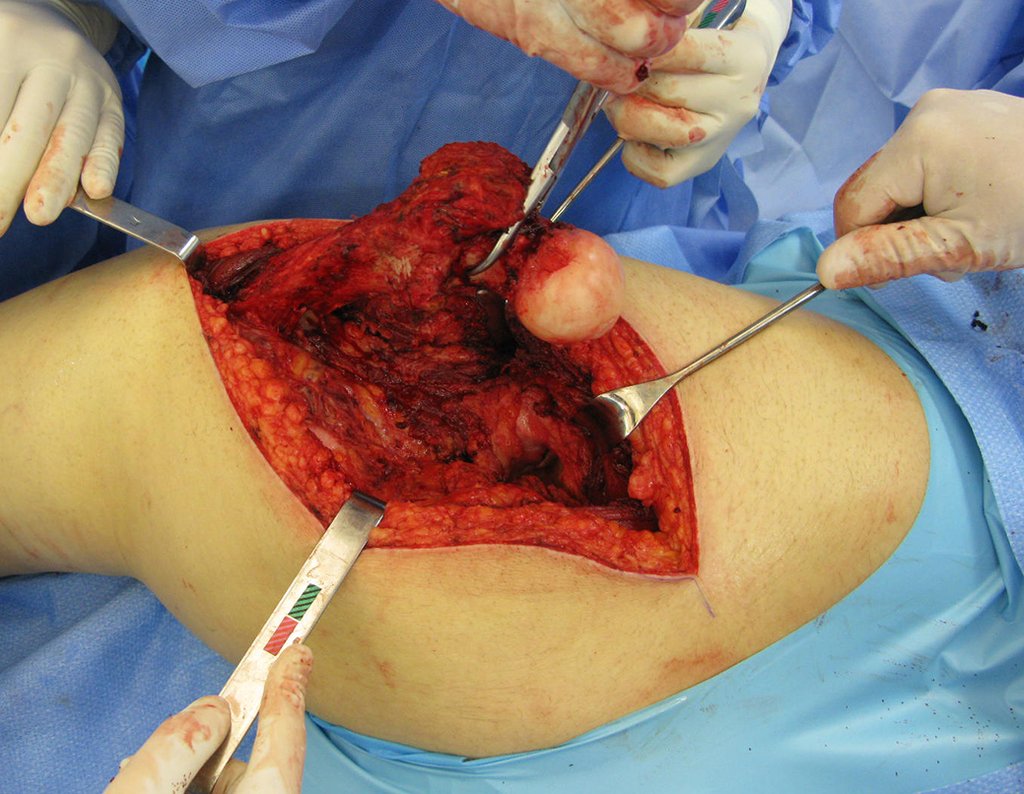
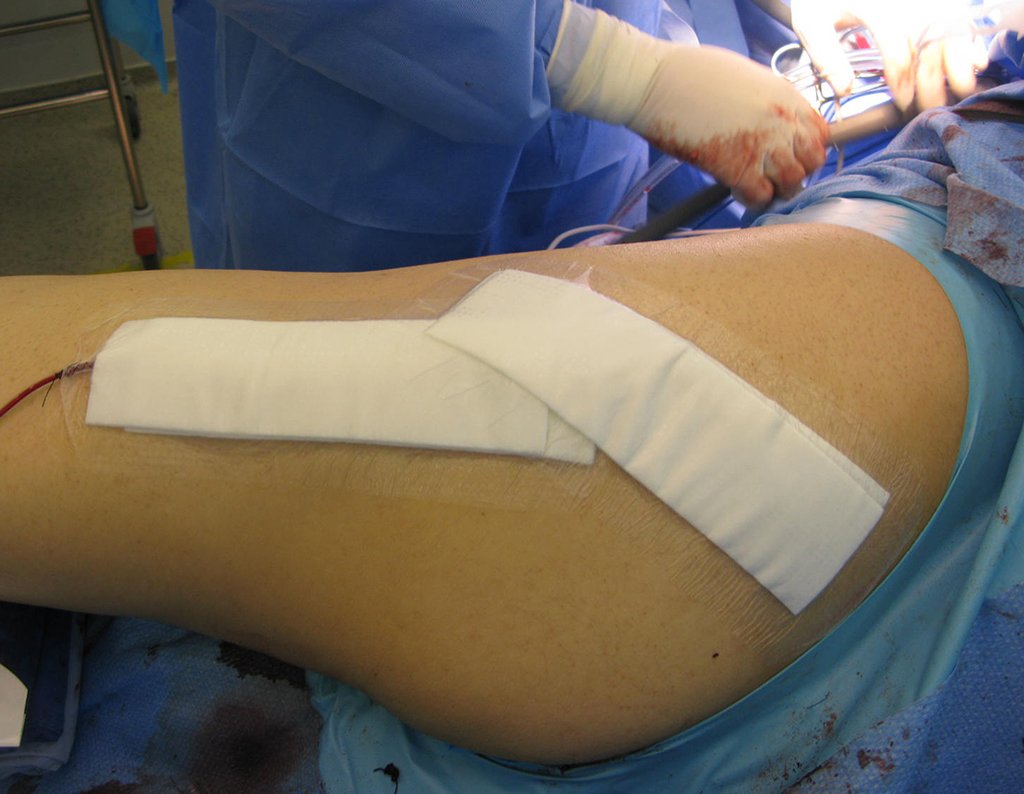
The surgery must be performed with caution, deepening the incision little by little , to achieve hemostasis in layers . Adequate anesthesia should not induce hypotension , as this is the only way the surgeon can properly observe the sectioned capillaries and make sure that he is performing an operation without blood loss, neither at that moment nor at a later time.
In oncological surgeries, the surgeon cannot have a “heavy” hand. The patient is already weakened by the illness, by chemotherapy, has possibly already undergone transfusions and the need for blood replacement must be avoided. Garroting should not be used except in amputation surgeries.
During anesthesia the patient cannot feel pain. It is not enough to be sedated, as if there is pain it increases the pressure, making hemostasis with electrocautery difficult.
Author: Prof. Dr. Pedro Péricles Ribeiro Baptista
Orthopedic Oncosurgery at the Dr. Arnaldo Vieira de Carvalho Cancer Institute
Office : Rua General Jardim, 846 – Cj 41 – Cep: 01223-010 Higienópolis São Paulo – SP
Phone: +55 11 3231-4638 Cell:+55 11 99863-5577 Email: drpprb@gmail.com