Osteoid Osteoma - Gammagraphy-guided surgery
Osteoid osteoma – Gammagraphy. A 23-year-old patient reports pain in the right thigh for three months, more intense at night and showing slight improvement with anti-inflammatories.
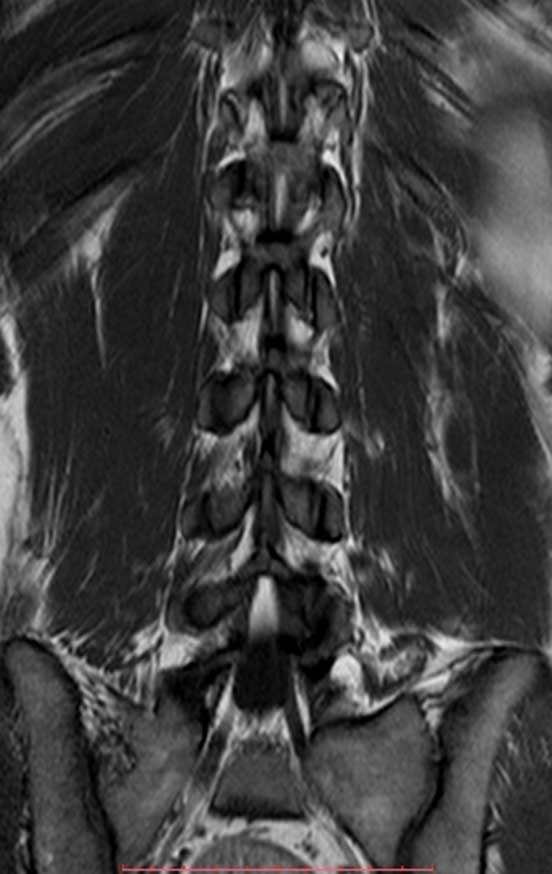
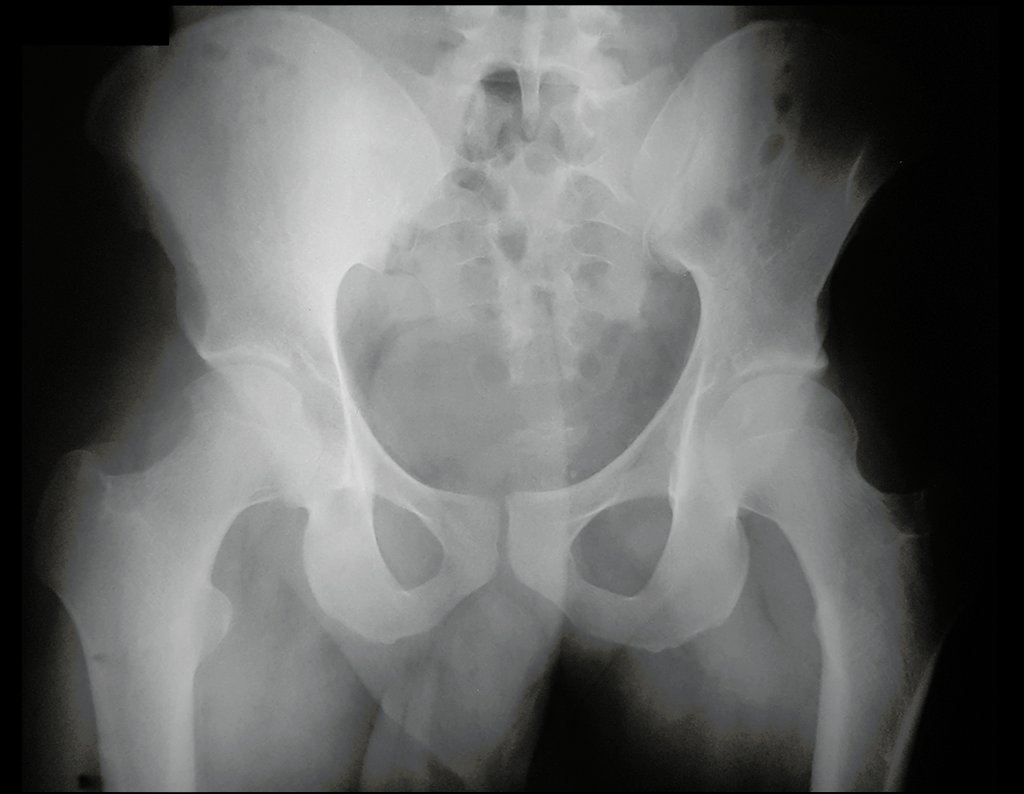
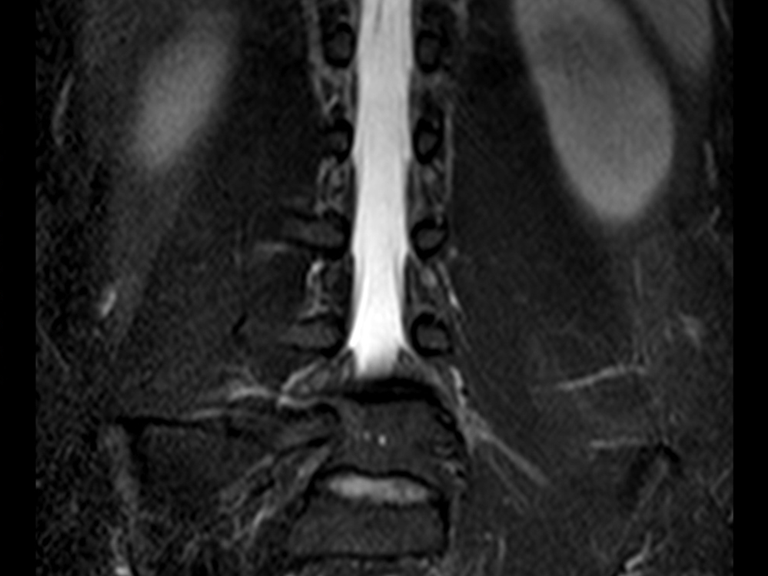
He underwent MRI of the lumbar spine and hip in April 2015, figures 1 to 8.
The MRI report at that time reported the diagnostic hypothesis of osteoid osteoma.
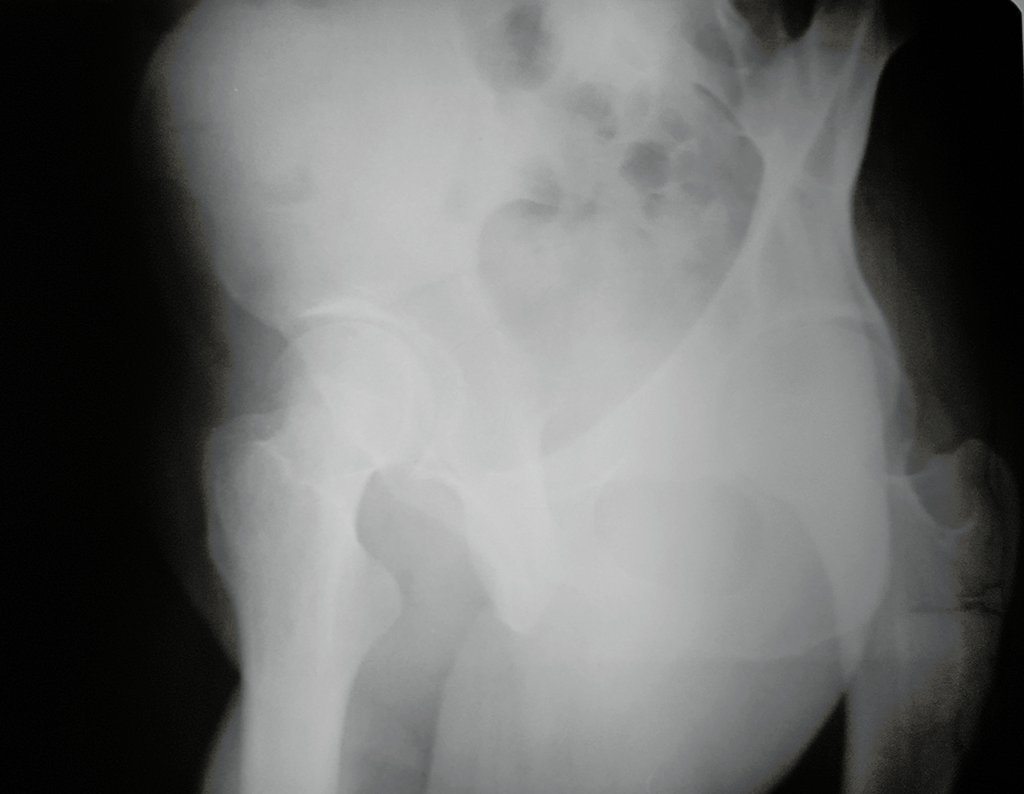
However, after four months, an arthro resonance imaging examination was performed in August 2015, which inferred the possibility of femoroacetabular impingement, figures 9 and 10.
Subsequently, an ultrasound was performed on September 6, 2016, and a new hip resonance was performed on September 22, 2016, figures 11 and 12.
The following month, in October 2016, a new hip resonance was performed, which reconfirmed the presence of the neoplastic lesion, arrows in red, in addition to inferring femoro-acetabular impact, figures 14 to 21.
On January 23rd, he sought our evaluation when we confirmed the clinical and imaging diagnosis of osteoid osteoma, advised him to repeat the imaging exams and planned the excision of the tumor for the July holidays, for the patient’s convenience, as the pain was controlled. and there was no urgency for this benign lesion.
The patient returns to his city of origin and undergoes imaging tests, as we had instructed, figures 26 to 30, which confirm the diagnosis of osteoid osteoma, already reported by this same laboratory in a previous exam, arrow in red and report highlighted in yellow .
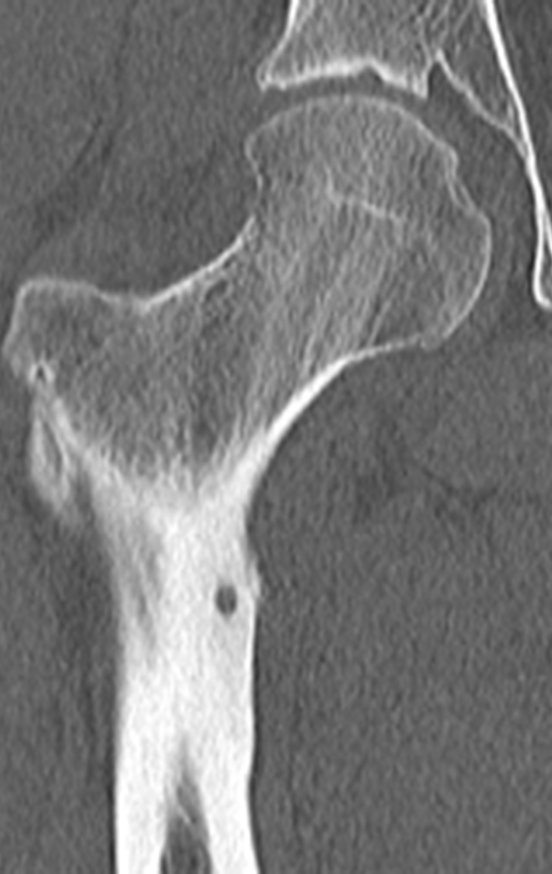
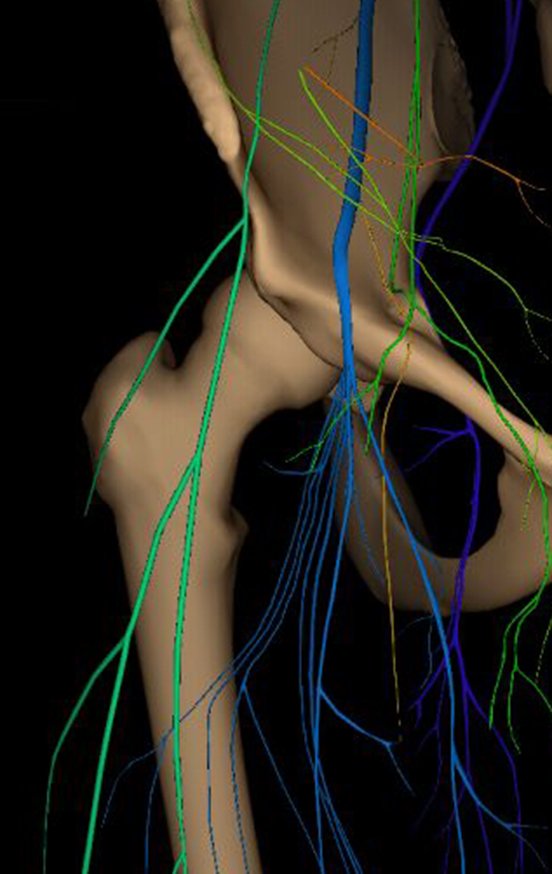
To plan this surgery, we reviewed the local anatomy, observing that the lesion was located in the intertrochanteric region, on the anterior surface of the femoral cortex, figures 31 and 32.
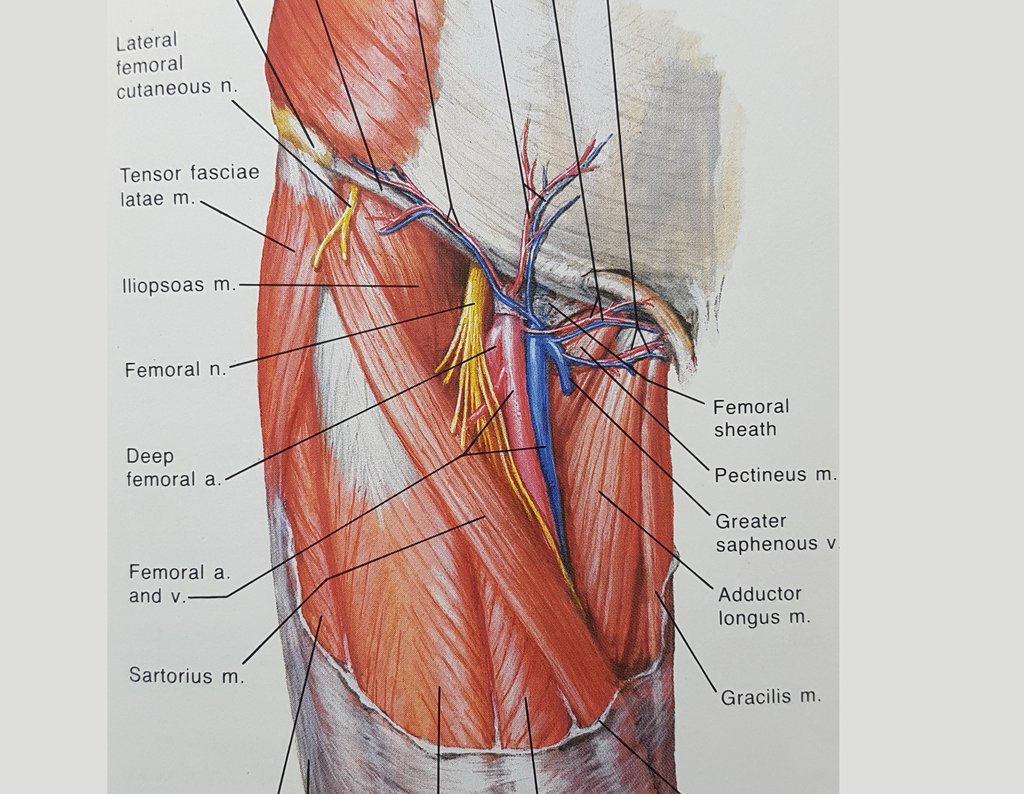
In another anatomical study, we can recall the muscles of this region and the extracapsular relationship of the lesion (figure 33), as well as their situation under the descending branch of the lateral femorocutaneous nerve and under the transverse branch of the anterior circumflex artery, white arrow, figures 34 and 35.
To safely resect the tumor niche, and avoid the need to place an autologous bone graft, we planned to use intraoperative gammagraphy, figure 36.
The day before surgery, technetium was injected into the patient and the center with the highest uptake of the isotope was marked with gamma probo.
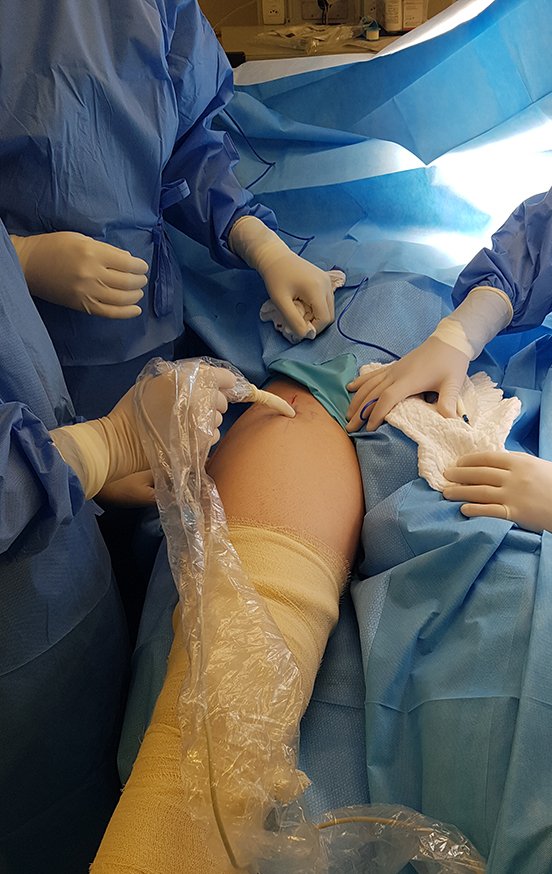
The next day, with the patient anesthetized, we checked the marking and during surgery we confirmed the location and size of the surgical incision, figures 37 to 39.
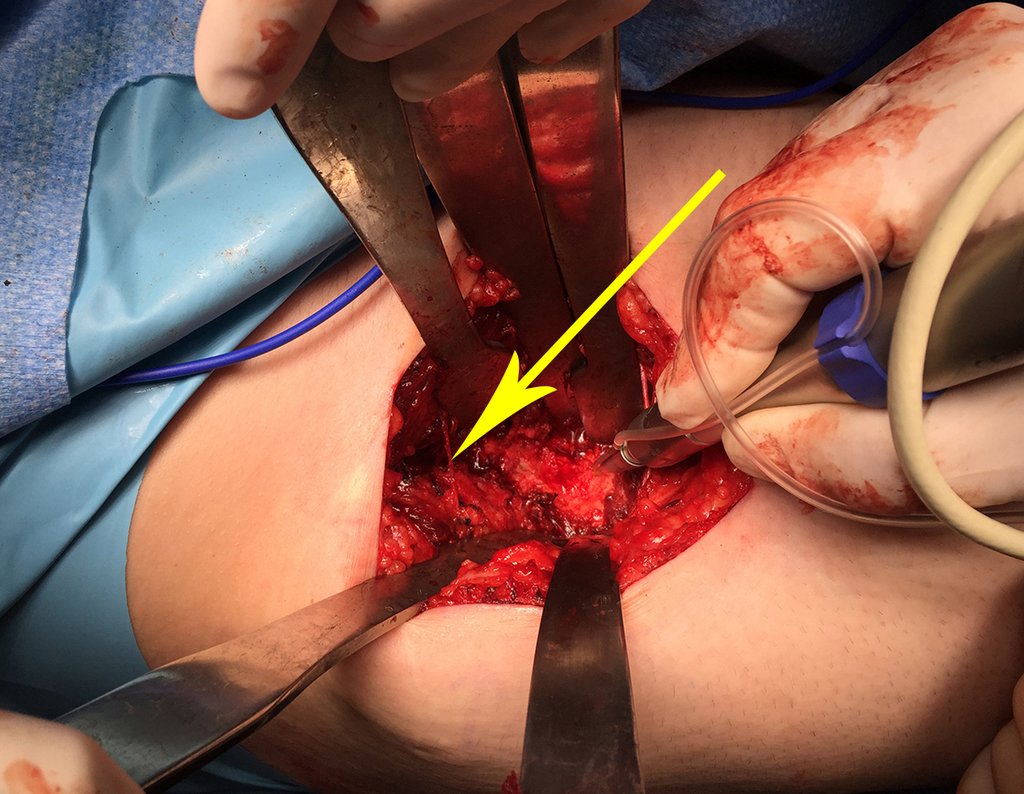
We sought to identify, isolate with a microdissector and protect the descending branch of the lateral femorocutaneous nerve, figures 40 and 41.
After operative dissection and preparation for tumor excision. we checked the center of the lesion using the gamma probe, directly on the bone cortex, videos 1 and 2.
Video 1 : Intraoperative check of the point of greatest uptake.
Video 2: Measurement of the intensity of technetium uptake by the tumor.
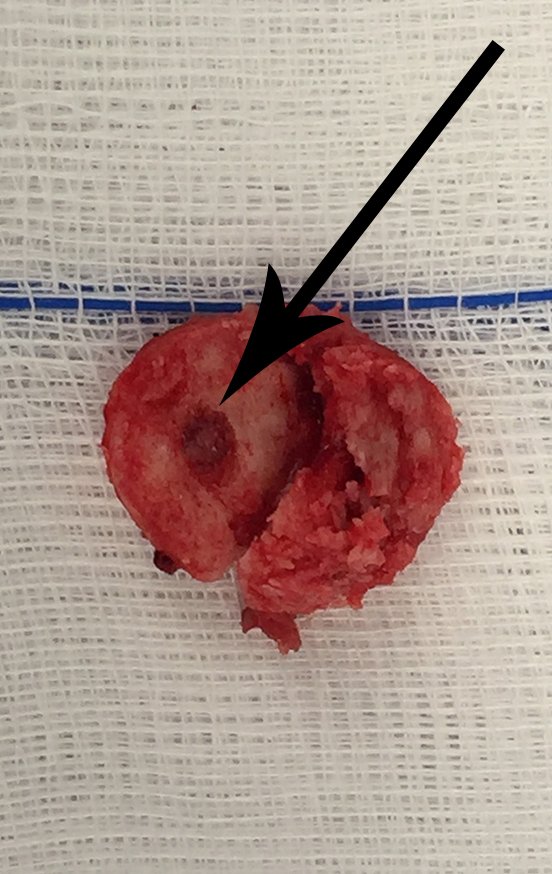
The yellow arrow highlights the nerve protected by the Hohmann retractor, the reading of the uptake peak and the circumferential cut of the cortex with a minimally invasive drill. We take care not to completely perforate the anterior cortex, avoiding weakening of the cortex and possible post-operative fracture, as well as eliminating the need for an autologous bone graft, reducing surgical morbidity. We resected the lesion, with a small circumferential margin of bone sclerosis, figures 42 to 46.
With the resected segment, placed in a vat, we checked the presence of the lesion through gamma probe capture, video 3.
Video 3 : After resection, the intensity of uptake in the resected piece is checked.
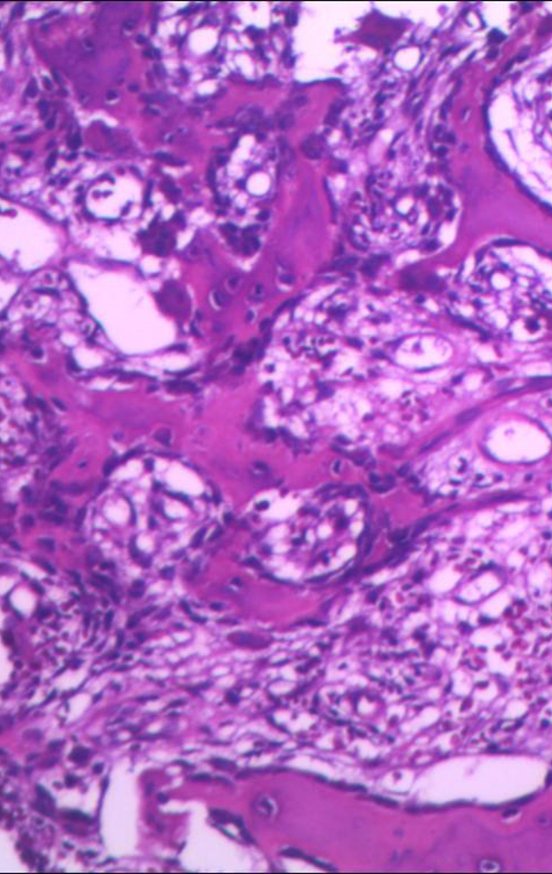
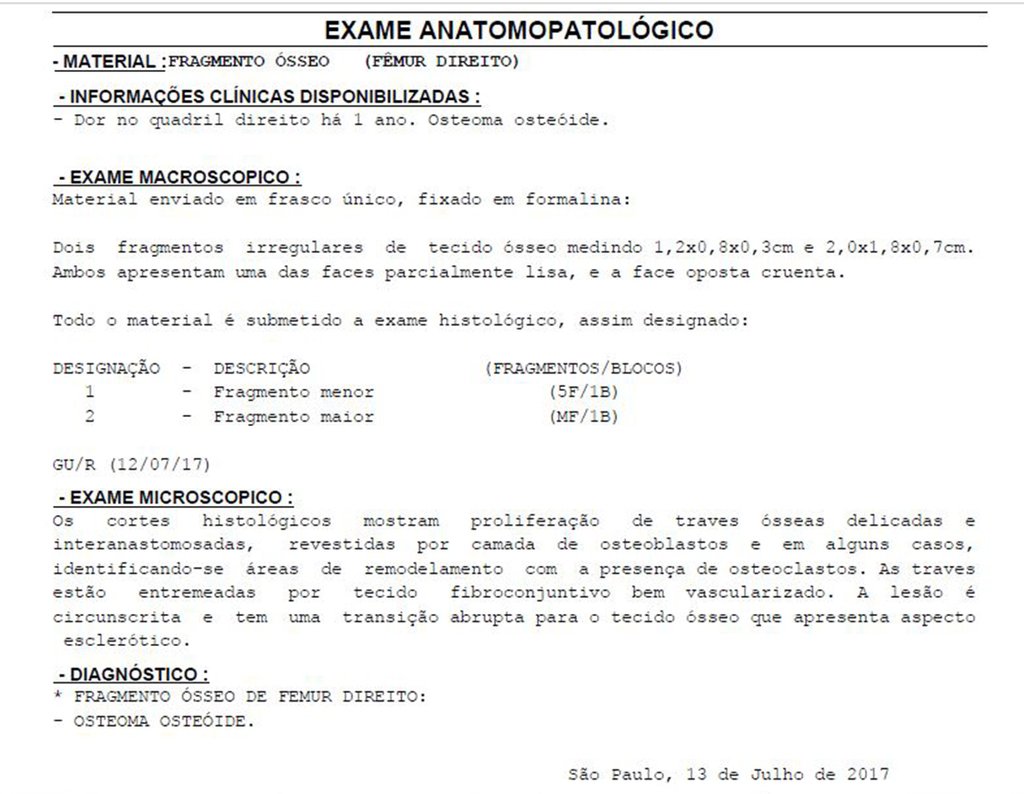
The anatomopathological study of the piece confirmed that it was an osteoid osteoma, figures 47 to 51.
Authors of the case
Author: Prof. Dr. Pedro Péricles Ribeiro Baptista
Orthopedic Oncosurgery at the Dr. Arnaldo Vieira de Carvalho Cancer Institute
Office : Rua General Jardim, 846 – Cj 41 – Cep: 01223-010 Higienópolis São Paulo – SP
Phone: +55 11 3231-4638 Cell:+55 11 99863-5577 Email: drpprb@gmail.com