Metastasis of Soft Tissue Sarcoma in the Sternum
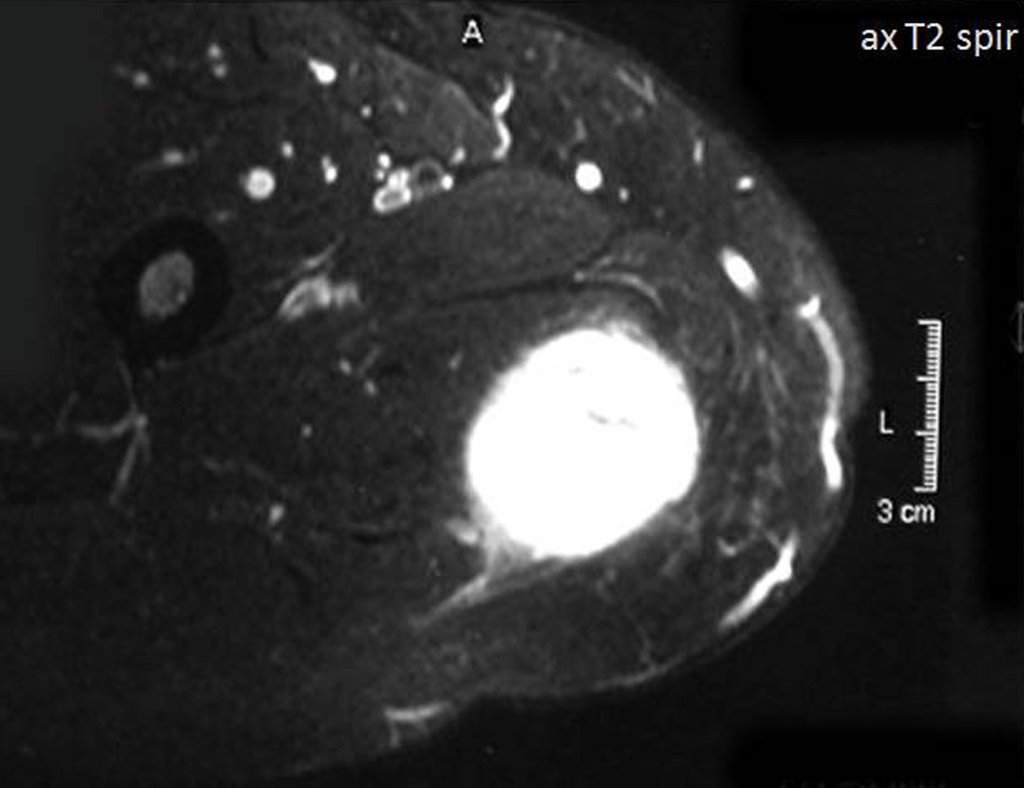
Metastasis of Sarcoma in the Sternum. Patient with pain and tumor on the medial aspect of the right thigh, affecting the soft tissues. The best imaging test to evaluate soft tissue injuries is magnetic resonance imaging (MRI). This examination revealed the presence of a solid lesion measuring approximately 5.5 cm by 7.0 cm, within the adductor muscles of the thigh (Figures 5, 6, 7, and 8).
With this clinical and imaging picture we have to answer the following question:
1- Perform a biopsy, or biopsy resection, since in this case it is possible to excise the lesion with an oncological margin, without changing its function?
2- If the biopsy result reports a lesion without atypia, fibroxanthoma for example, would surgical resection change? Would a resection be more economical? Even with the characteristics of these images, since the biopsy is only a small sample of the lesion?
For us, conduct cannot change . Resection must be performed with an oncological margin in this case. The biopsy is performed with the aim of guiding neoadjuvant therapy if it is confirmed to be a sarcoma.
3- What type of biopsy to perform? With Tru-cut needle? Incisional? With diagnosis by freezing and management?
It is best to always perform a biopsy of soft tissue lesions with a tru-cut needle, guided by ultrasound and frozen pathological anatomy, to validate the material collected. The resection procedure will depend on each case and can be performed in cases where the oncological margin is possible, without functional loss. Otherwise, you must wait for the final result of the anatomical pathological examination, including immunohistochemistry, when necessary.
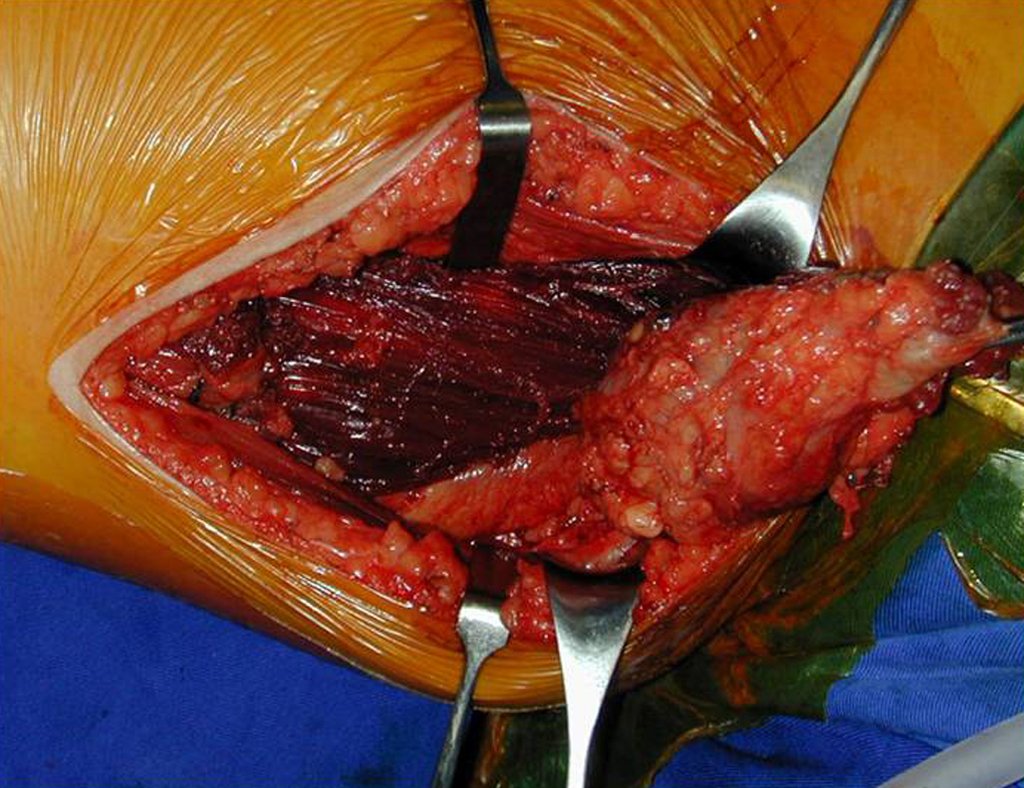
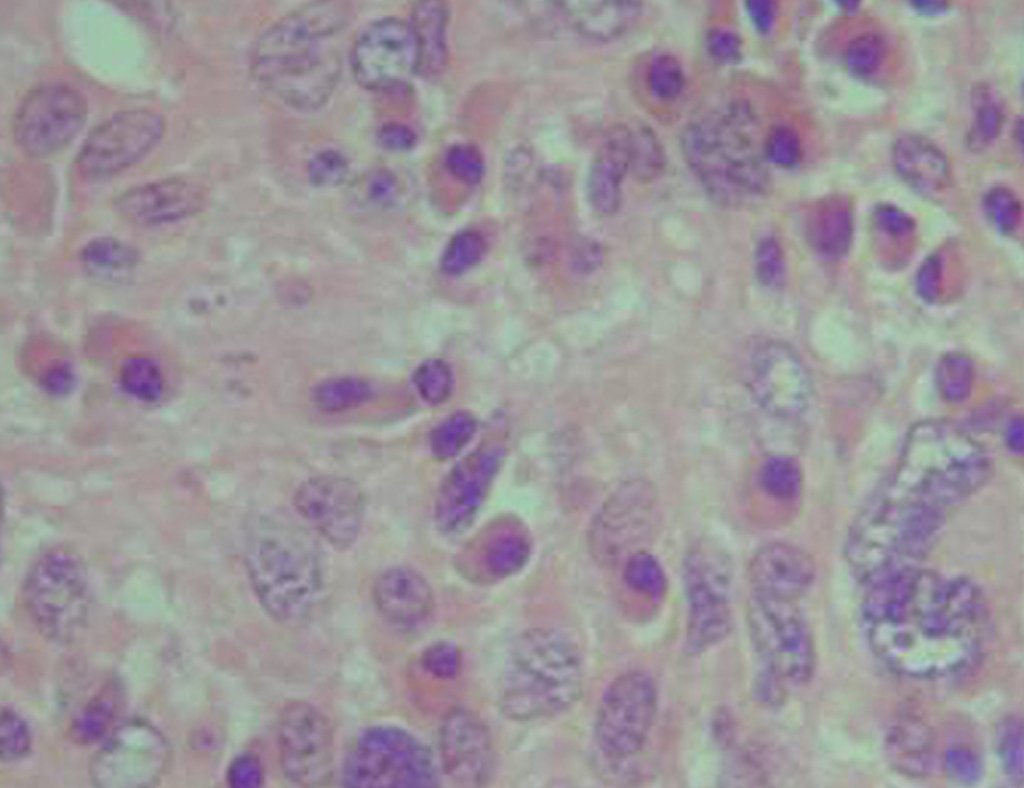
In this case, the diagnosis was high-grade sarcoma , on frozen section and as resection with a margin was possible, the surgery was performed under the same anesthesia (Figures 9 and 10). We can observe the histological aspect of this tumor (Figures 11, 12 and 13).
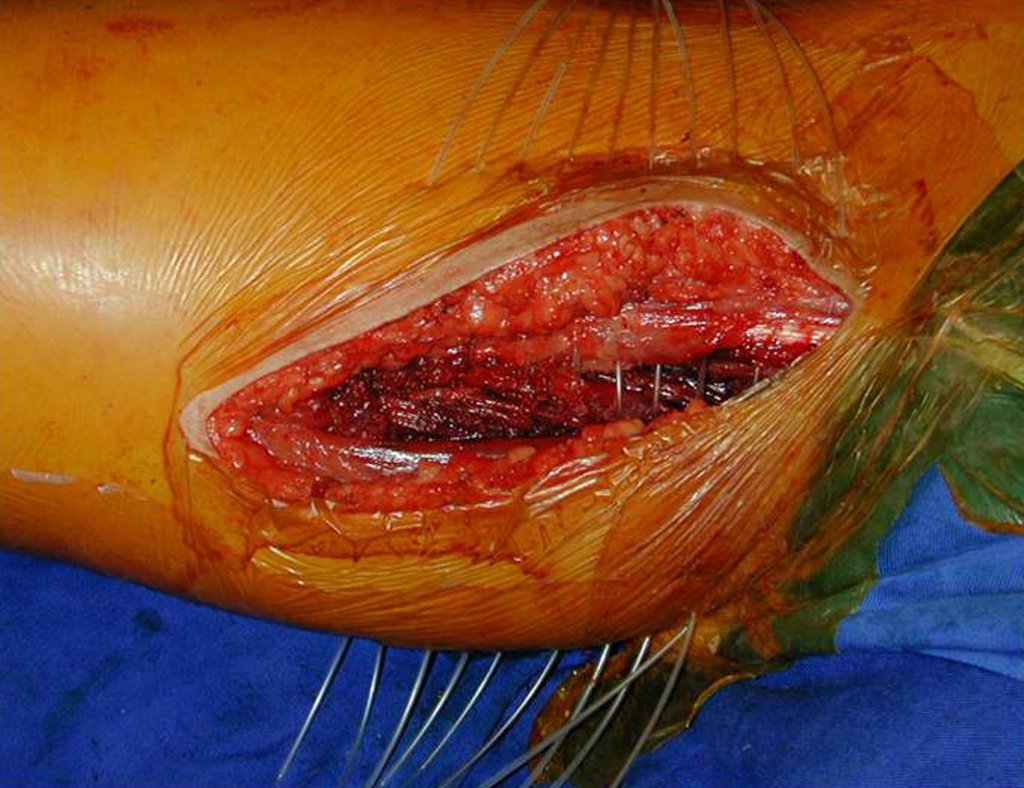
Adjuvant radiotherapy was initiated during surgery with the placement of catheters for brachytherapy. These catheters are passed through a needle with sufficient tubular diameter. The catheters are passed and secured with a plastic clip (Figures 14 and 15). The catheters are subsequently removed and completed with external radiotherapy. Figure 16 shows the local appearance after treatment.
The case was discussed in a multidisciplinary team that chose not to perform adjuvant chemotherapy, as the relationship between possible benefits and side effects was not favorable, taking into account the fact that it does not increase overall survival, Lancet 1997 1 .
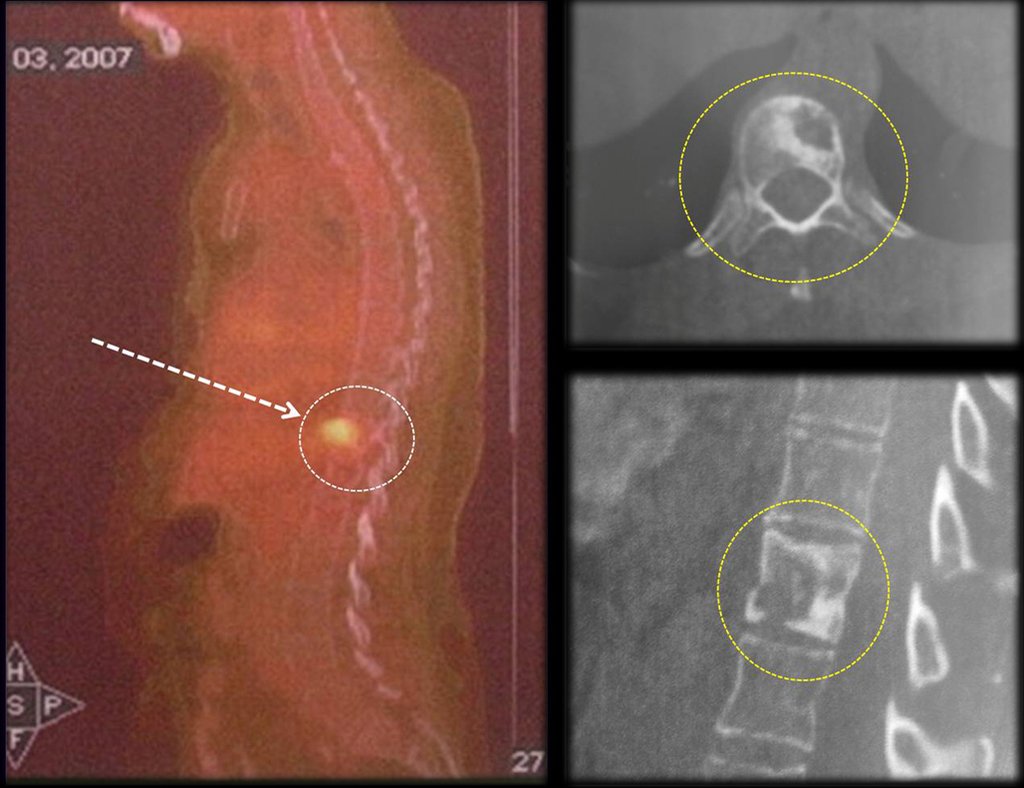
In the control evaluation, after four years and three months, the patient reported bulging and pain in the sternum. The lateral x-ray highlights the presence of a lesion in the distal portion of the sternum (Figures 17 and 18). The bone scintigraphy performed for skeletal staging revealed a single lesion, with intense uptake in this region (Figures 19 and 20).
With this patient’s clinical history and these images, we asked the following question:
1- Would you take a biopsy of this lesion?
2- What should we do if we are diagnosed with benign fibrohistiocytoma? or aneurysmal bone cyst? What is the conduct? Curettage? With this patient’s history of high-grade sarcoma? The biopsy is just a sample, so it is questionable to repeat the biopsy until a diagnosis of a malignant lesion is made in this patient. We must assume the need for resection with margin of this lesion, since it is the oncological approach and resection of the sternum does not cause aesthetic or functional damage. Neoadjuvant chemotherapy would not add any advantages to the case, nor would prior radiotherapy change the need for resection of the affected area.
3- If we are going to resect the sternum, what access route should we use? What is the incision that allows wide and more aesthetic resection?
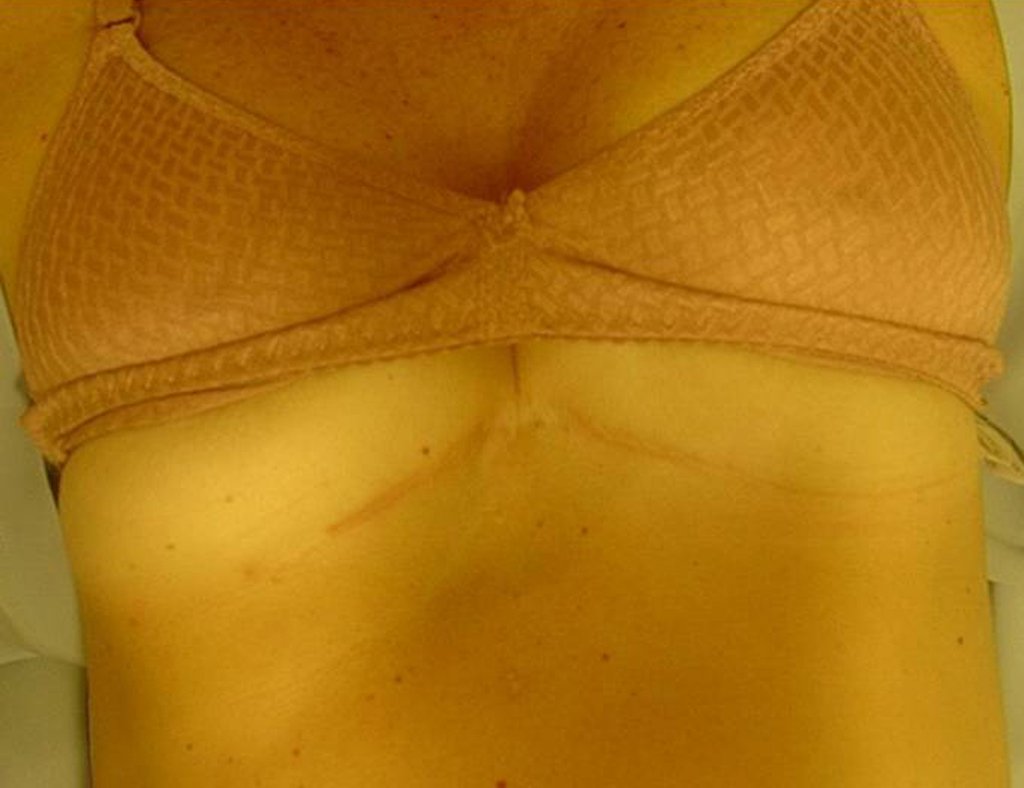
We chose the horizontal, inframammary route (Figures 29, 30, 31, 32 and 33).
This case was discussed again in a multidisciplinary meeting. Postoperative radiotherapy was not indicated after the type of en bloc resection in this case, in this location. The literature review at the time did not indicate the benefit of chemotherapy versus toxicity and the evidence of overall survival was equal, Cancer 2008 2 . The patient does not receive adjuvant treatment.
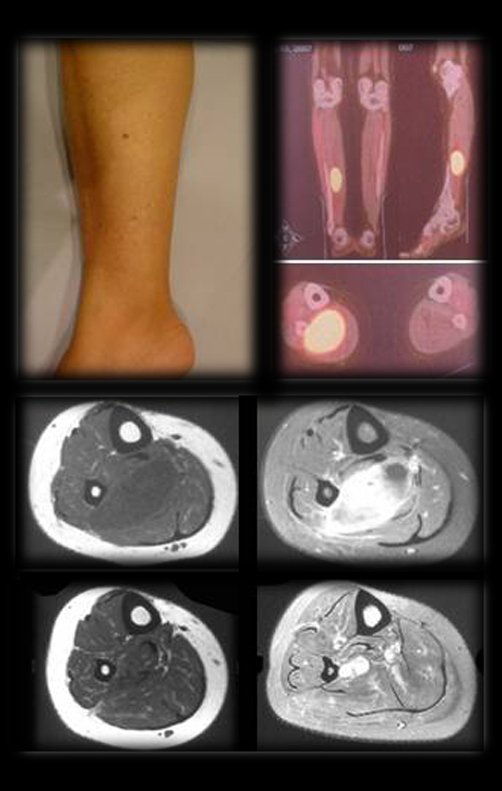
After seven years and eight months of treatment for the thigh tumor and four years and two months of resection of the sten, the patient presented metastasis in the right calf (Figures 36 and 37) and the staging revealed a lesion in the thoracic vertebra, T11 and in the retroperitoneum (Figures 38 and 39).
A randomized study in 2012 of adjuvant chemotherapy with doxorubicin and ifosfamide did not show any benefit in relapse-free survival or overall survival, Lancet 3 . In the literature in 2013, the benefits of neo-adjuvant and adjuvant chemotherapy, UpToDate 4, are still uncertain .
Chemotherapy for soft tissue sarcoma still needs to evolve a lot. The vast majority of treatments for these sarcomas are not individualized. We know that chemotherapy for Ewing sarcoma is different from chemotherapy for osteosarcoma, which is why we have favorable results in the treatment of these conditions. The large pool of sarcomas cannot be placed on the same treatment protocol. Just as work on the outcome of treatment of this pool of tumors does not allow conclusions. Cases that respond are mixed with the majority that do not respond and the chemotherapist is left without parameters. In adults, toxicity is worsened by comorbidities and the cost/benefit ratio is unfavorable.
Surgery is still the primary treatment for soft tissue sarcomas. Radiotherapy has its role in controlling high-grade sarcomas, but it does not rescue an inadequate resection. Neoadjuvant radiotherapy has fewer complications for the surgical wound than brachytherapy. Adjuvant radiotherapy is recommended in practically all cases, with the exception of superficial lesions, resectable tumors smaller than five centimeters and lesions of low histological grade, UptoDate 5 .
Bibliographic references
1.Adjuvant chemotherapy for localized resectable soft tissue sarcoma of adults: meta analysis of individual data, Lancet 1997; 350:1647-54
2.Adjuvant and neoadjuvant chemotherapy
3.Local treatment for primary soft tissue sarcoma of the extremities and chest wall
4.Treating soft tissue sarcomas
Authors of the case
Author: Prof. Dr. Pedro Péricles Ribeiro Baptista
Orthopedic Oncosurgery at the Dr. Arnaldo Vieira de Carvalho Cancer Institute
Office : Rua General Jardim, 846 – Cj 41 – Cep: 01223-010 Higienópolis São Paulo – SP
Phone: +55 11 3231-4638 Cell:+55 11 99863-5577 Email: drpprb@gmail.com