Tibial Growth Plate Autotransplantation
Summary
Tibia autotransplantation. Bone injuries, which occur in the proximal region of the tibia in children, can affect the growth physis and represent a challenge for orthopedic treatment to reconstruct the bone defect created. Reconstruction methods do not always consider the bone growth potential of this segment. The objective of this work is to present a new surgical technique for bone reconstruction, based on the transposition of the ipsilateral fibula, with its growth physis, without the need for a microsurgical technique and using a sliding internal fixation device. Material and Method: The authors report two cases of patients with bone sarcoma in the proximal region of the tibia, with involvement of the growth cartilage, which were treated using the proposed technique. Results: In both cases there was bone consolidation, hypertrophy and longitudinal growth of the transposed fibula. Conclusion: The technique, proposed by the authors, maintained the vascularization of the auto-transplanted bone segment, without the need for microsurgery and preserved the potential for physeal growth. The implant used allowed longitudinal growth of the bone, evidenced radiographically. Level of evidence IV. Case series.
Descriptors: growth, sliding, device, fibula, physis, fixation, tibialization sarcoma, transplant.
Abstract
Bone lesions, which occur in the proximal tibia in children, can affect the growth plate and represent a challenge to the orthopedic treatment for reconstruction of bone defects created. Reconstruction methods do not always compensate the potential for bone growth in this segment. The objective of this paper is to present a new surgical technique of bone reconstruction, based on the transposition of the ipsilateral fibula with it’s growth plate, without the need for microsurgical technique and using a sliding internal fixation device. Material and Method: The authors report two cases of patients with bone sarcoma at the proximal tibia, affecting the growth cartilage, which were treated by the proposed technique. Results: In both cases, there were bone healing, hypertrophy and longitudinal growth of the transposed fibula. Conclusion: The technique proposed by the authors, kept the vascularization of bone segment auto-transplanted without the need for microsurgery and preserves physeal growth potential. The implant used allowed the longitudinal bone growth, as radiographically seen. Levels of evidence IV. Case series.
Keywords: growth, sliding, device, fibula, physis, fixation, sarcoma, tibialisation, transplant.
INTRODUCTION
The proximal third of the tibia, in the skeletally immature population, houses a growth physis responsible for approximately 30% of the final length of the limb in adulthood 1 . This region is also the second most common location for the incidence of primary bone tumors, behind only the distal third of the femur 2 .
Tumors that arise in the proximal region of the tibia, before skeletal maturity, can affect the growth physis and generate discrepancies in the final length of the lower limbs. The same can occur after trauma or secondary to diseases such as osteomyelitis, in this region.
Tumor resection, in this segment of the tibia, requires reconstruction of the bone defect generated, which, due to young age, may have unsatisfactory results with traditional methods, recommending amputation as an alternative 3 (figures 1a, 1b and 1c).
Among the reconstructions of this segment, we can mention replacement with a non-conventional endoprosthesis, use of homologous or autologous grafts and bone transport 4-8 .
None of the above methods replace the injured growth physis. Bone transport, which allows longitudinal stretching of the bone, may be unfeasible due to the need for prolonged use of an external fixator. In cancer patients, who are immunosuppressed by adjuvant chemotherapy, the fixator favors infections, in addition to requiring multiple surgeries to equalize the limbs in young children.
The use of the vascularized fibula to fill bone defects gained great momentum with the development of the microsurgical technique, as it allows the use of the contralateral fibula 9-13 .
The use of the proximal segment of the fibula with its physis, using a microsurgical technique, made it possible, for the first time, to reconstruct bone defects and reestablish the longitudinal growth of the bone 14 . However, it requires a specialized team, as well as special and high-cost materials and equipment, and is subject to several complications.
The objective of this work is to present a new surgical technique for reconstructing bone injuries that compromise the proximal region of the tibia and its growth plate in children. This technique consists of transposing the ipsilateral fibula, together with its growth physis, preserving blood supply and the capacity for longitudinal growth, without the need for a microsurgical technique, using a single surgical access route. We describe two cases where this technique was used.
MATERIAL AND METHOD
The medical records of two patients with bone sarcoma in the proximal region of the tibia, who presented growth cartilage impairment, were retrospectively analyzed and were treated surgically using the technique of autotransplantation of the proximal segment of the fibula to the tibia, developed by the co-author. Baptista*, in the Department of Orthopedics at Santa Casa de São Paulo.
Description of the surgical technique
The patient is positioned in a horizontal supine position. A single, curved access route is used, starting above the proximal tibio-fibular joint, descending anteriorly along the tibial crest and curving medially, a few centimeters below the location where the fibular osteotomy will be performed (figure 2a) .
The tibialis anterior muscle is exposed, the perimysium is opened and the muscle is moved laterally, leaving the inner layer of this perimysium adherent to the periosteum, aiming to preserve the oncological resection margin of the tibia (figure 2b).
The neck of the fibula is identified and the common peroneal nerve isolated. The proximal tibiofibular joint is approached and the joint capsule, together with the anterior ligament, posterior ligament, arcuate popliteal ligament, fibular collateral ligament and the biceps femoris muscle tendon are released (figure 2c).
The proximal epiphysis of the tibia together with the anterior tuberosity are isolated from the metaphyseal region (figure 2d).
A Kirschner wire is passed through this epiphysis, horizontally, at the point where the proximal fixation will be made and the position of the plate is then verified (figure 3a).
The segment to be resected is measured, following the oncological margin, and the distal osteotomy of the tibia is performed, in the diaphyseal region. The posterior muscles of this portion are disinserted to the proximal epiphyseal region. The tibial epiphysis is then separated from the tumor by transepiphyseal osteotomy, preserving as much of the epiphyseal bone with its articular cartilage as possible and the tumor is resected (figures 3b and 3c).
To replace this bone defect, the proximal segment of the ipsilateral fibula is used, which is isolated from the tibiofibular joint and the lateral collateral ligament. A small deperiostization is performed, of one to two centimeters, at the height where the osteotomy will be made in the fibular shaft (figure 4a). After the osteotomy, this deperiostized part is nailed into the tibial diaphysis (figure 4b).
The proximal segment of the fibula, together with all the muscles and its nourishing arteries, is transferred to the center of the remaining tibial epiphysis and the fibular collateral ligament is inserted into the periosteum of the tibia (figure 4c).
Osteosynthesis with screws is performed using the Baptista extensible internal fixation device, placed on the medial side of the leg, which is previously custom-made for each case 15 (figure 4d).
The transferred fibula is interposed between the tibial epiphysis and the distal portion of the tibia.
Subsequently, soft tissue reinsertion, hemostasis, placement of an aspiration drain and closure in layers are performed. After dressing, the limb is placed in an orthosis that was also previously made to measure.
This orthosis is used as an external protective support, until the fibula consolidates and increases its thickness, to be able to support the load (figure 6). This period can vary from 3 to 8 months.
Case 1
Male patient aged 12 years and 11 months, presenting with osteosarcoma in the proximal region of the right tibia. He underwent wide resection of the tumor, preserving the proximal epiphysis of the tibia. The proximal portion of the fibula with its physis was translated medially to the tibial epiphysis, preserving its blood supply, and osteosynthesis was performed with the sliding fixation device.
After surgery, the limb was kept in an orthosis and weight-bearing was started in the fourth month after surgery, when there were radiographic signs of consolidation. Orthopedic and oncological follow-ups were carried out and the patient returned to his usual activities, with bone consolidation and fibula hypertrophy observed.
Case 2
A 2-year-old, 7-month-old male patient with Ewing’s sarcoma in the proximal region of the right tibia underwent resection of the metadiaphyseal segment, compromised by the tumor, preserving the proximal epiphysis of the tibia. The proximal portion of the fibula with its physis was translated medially under the tibial epiphysis maintaining its blood supply and osteosynthesis was performed with the extensible internal fixation device. In this second case, the proximal plate was improved by creating a support to support the remaining tibial plateau, with the aim of increasing stability and avoiding angular deviations (figure 7: a and b). In the distal plate, grooves were made every three mm to facilitate observation of sliding, documenting the growth of the fibula. After surgery, the leg was kept in an orthosis, specially made for the case, and weight-bearing began in the third month after surgery. The patient continued with adjuvant chemotherapy and, in the fourth month, began walking without protection. Bone consolidation and hypertrophy of the fibula can be seen in figure 7: c and d.
RESULTS
In the first case, the screws were not locked, which is why valgus deviation occurred, confirmed radiographically, by tilting the screws and clinically (figure 8. abc). This patient underwent the first scanometry of the lower limbs one year after surgery, observing growth of the fibula of 0.75 cm and the reorientation of the screws (figure 8. b).

The spontaneous correction of this angular deviation, observed clinically, and the horizontalization of the screws, documented radiographically, allows us to confirm the growth of the fibular epiphysis and the sliding of the device. The second scanogram shows growth of 1.2 cm (figure 8. de). The second scanometry was performed at 2 years and 2 months postoperatively, and it was possible to observe growth of 1.2 cm in the transposed fibula segment (figure 8. f).
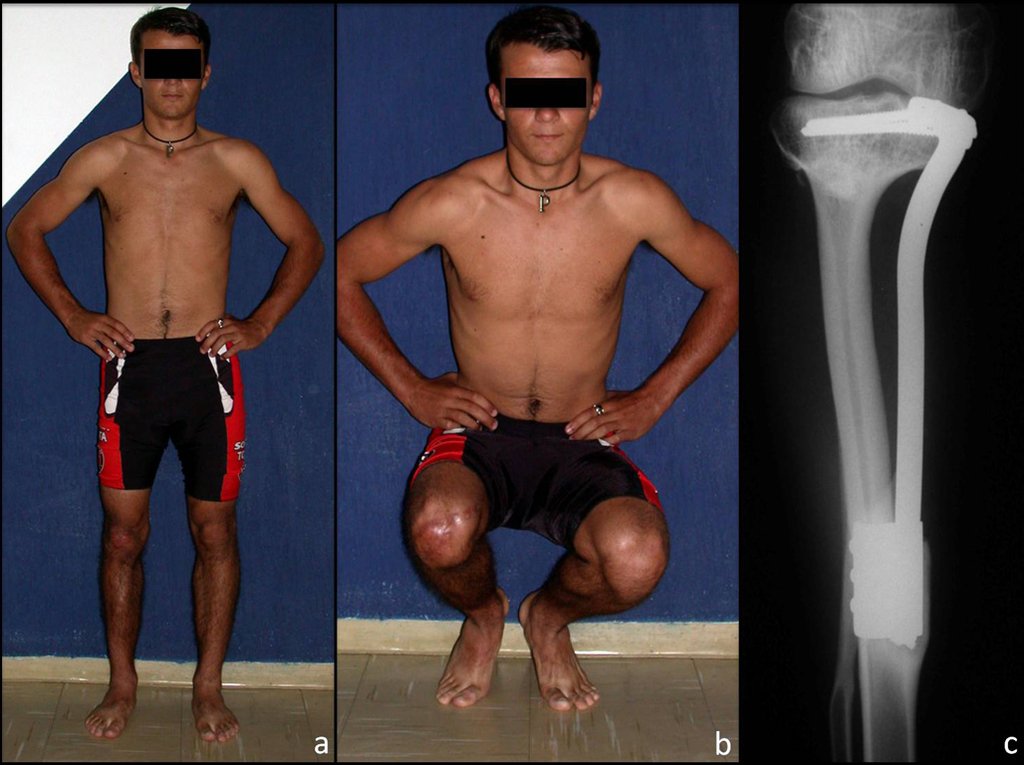
The patient is a cattle herd, 26 years old, with 14 years of evolution, without recurrence of the disease, with equalized limbs and functionally well (figure 9).
The Patient in Case 2 showed growth of approximately 0.3 cm in the transposed fibula in the first eight months after surgery, a period in which there was orthopedic follow-up.
After this period, the patient in Case 2 showed growth of approximately 0.3 cm in the transposed fibula in the first eight months after surgery, a period in which there was orthopedic follow-up.
The patient died during adjuvant treatment due to chemotherapy complications.
DISCUSSION
Reconstruction of bone defects in the proximal region of the tibia represents a challenge for the surgeon, especially when these lesions compromise the growth cartilage.
Transfemoral amputation is sometimes indicated as the method of choice in young children, due to the difficulty in reconstruction and the discrepancy in the final length of the lower limbs. The use of a prosthetic orthosis, adapted to the amputation stump, can allow early rehabilitation and walking autonomy.
This method, however, is linked to an important psychological and social impact, due to the mutilation of a large joint, with increased energy expenditure for walking.
Difficulties in adapting to the use of prosthetic orthoses, their high cost and the need to acquire new prostheses as the patient grows are other negative aspects of amputation.
Reconstruction of the proximal segment of the tibia with a non-conventional endoprosthesis is an alternative for these patients. It allows limb preservation and early ambulation. However, high rates of complications related to the method are reported, such as infection, aseptic loosening, mechanical failures, fractures and limitations in physical activities, among others 4,5,16 .
Another important point to be highlighted is the growth of the limbs, both in the operated limb, causing difficulties in adapting the endoprosthesis, and in the opposite limb, causing a discrepancy in length between them. Jeys and collaborators report a series of 661 patients who underwent non-conventional endoprosthesis to treat bone tumors, among which 42% had implant failure within 10 years of follow-up 17 .
Exposure of the endoprosthesis in the proximal third of the tibia and consequent infection are related to the difficulty in covering the implant by the soft tissues in this region.
Wang and collaborators report the use of gastrocnemius and soleus flaps to cover the prosthesis in 11 patients with the aim of avoiding such complications. There were, however, 3 cases of discrepancy between the lower limbs that were treated with contralateral epiphysiodesis 18 .
The technique of transferring the fibula together with its growth physis presents slower rehabilitation, especially when starting to walk, as it is necessary to wait for bone consolidation between the tibia and the transposed fibula. Subsequently, hypertrophy of the fibula occurs, observed in the cases presented, which makes this segment resistant.
This method represents a biological reconstruction and, therefore, once consolidation and hypertrophy occur, we can consider it as a definitive reconstruction method, performed with a single surgical intervention.
Preservation of the proximal epiphysis of the tibia allows the articular surface of the knee to be maintained, this represents an advantage of the method when transepiphyseal osteotomy can be performed and is also a necessary condition for its use.
The longitudinal growth capacity of the transposed fibular segment, observed in the cases presented, is also an important factor to be mentioned, as it can avoid or minimize lower limb discrepancy.
Bone grafts can also be used to treat this type of injury, especially in cases where the tibial epiphysis can be preserved, which occurs in up to 20% of cases 19 .
However, as it is not a vascularized bone, the graft may not undergo integration and the method is subject to failure, which may result in high rates of fractures or non-union, in addition to not solving the problem of discrepancy 20 . It is difficult to obtain a large quantity of autologous grafts in young children and homologous grafts present greater difficulty in integration, in addition to antigenicity.
Weitao and collaborators reported the use of allografts in 15 patients with bone sarcomas, with a mean age of 11.75 years (seven to 24) in the distal region of the femur or proximal region of the tibia, where the epiphysis of the bone could be preserved. The results were growth discrepancy in 4 patients, delayed consolidation in 15 patients, graft rejection in 2 cases, infection in 1 case and breakage of the synthesis material with partial reabsorption of the graft in 1 case 7 .
Campanacci and collaborators reported the use of osteoarticular allograft in the reconstruction of osteosarcomas of the distal femur and proximal tibia in which the articular surface was preserved with the tumor. At the end of 5 and 10 years of follow-up, the survival rates of grafts placed in the tibia were only 45% and 20% respectively, with better results in the femoral region 6 .
The advantages of transposition of the fibula over the graft are the fact that it is an osteo-muscular flap, that is, it has vascularization and bone “turn over”, actively participating in consolidation, in addition to maintaining the growth potential of the physis.
As can be seen in figures 7 and 8, the fibula undergoes progressive hypertrophy, a fact that increases its resistance, unlike allografts, which can fail even after years of integration 12,21 .
Distraction osteogenesis with an external fixator can be considered as a reconstruction option. It does not require the use of a graft and makes it possible to fill the bone gap created by tumor resection through bone transport, however, it requires prolonged use of the external fixator and the risk of infection 22 .
Fang and collaborators 8 reported three cases of osteosarcoma in children aged 10 to 14 years, one in the distal region of the femur and two in the proximal region of the tibia, who underwent tumor resection and installation of an Ilizarov apparatus, following the principle of osteogenesis by distension. One of the patients required re-surgical intervention due to loss of bone alignment. Two patients achieved union within eight months, and the fixator was removed. The third patient underwent compression and redistension to achieve bone consolidation. Two cases presented infection along the path of the fixator wires. At the end of two years of follow-up, the lower limb discrepancy was 1.0 to 1.5 cm.
The use of the ipsilateral fibula for the treatment of tibial injuries, especially in the aftermath of trauma, emerged in 1884, when Hahn described its use for the treatment of tibial pseudarthrosis 11 .
Several techniques have been described and the occurrence of bone consolidation, with fibular hypertrophy, established this method as an alternative in the treatment of tibial defects 9,10,11,12,23,24,25,26,27 .
However, these studies did not use the epiphyseal region of the fibula, limiting its use primarily to diaphyseal injuries of the tibia.
The development of microsurgery has brought a new dimension to the treatment of bone defects.
Taylor and colleagues described the use of the fibula to restore segmental defects in several bones other than the tibia, using microsurgical vascular reconstruction of the fibula 13 .
However, the need for a specialized surgical team, the high cost and the possibility of postoperative arterial or venous thrombosis in microsurgery constitute important disadvantages in relation to techniques that do not require microsurgery 28,29 .
Microsurgery, however, paved the way for the possibility of including the physis in a bone flap, preserving its blood supply and consequently the potential for longitudinal growth of the bone segment 14,30 .
However, the first reports of growth cartilage transposition presented inconsistent or poor results, with Straub reporting some bone growth in his work in 1929, as fragments of cartilage with or without bone were used in the form of grafts 31-34 .
Based on the description by Pho and collaborators 14 , several studies using a microsurgical technique of transposition of the fibula with the proximal physis and epiphysis showed that the growth potential of the physis could be preserved 35-38 . These studies, however, involve a microsurgical technique for the vascular reconstruction of the fibula, with the disadvantages and complications that the method presents.
The technique presented in this work has the advantage of not requiring any method to reconstruct the vascularization of the fibular segment, with its physis and epiphysis, as the vessels are preserved, as it is a local flap that is positioned under the tibial epiphysis.
We observed bone consolidation, fibular hypertrophy and longitudinal growth of the transplanted segments in the 2 cases presented. In the first case, the patient presented a valgus deformity of the right knee in the immediate postoperative period. With the growth, which occurred in the first year, there was correction of both the valgus deformity and the inclination of the screws, signs of growth of the fibular segment. Over the next fourteen months, the transposed fibular segment grew by another 1.2 cm (Figure 7). This patient, who was a teenager, 12 years old at the time, is now 26 years old, is functionally well and has equalized lower limbs.
In the second case, a child approximately three years old, who could have been ideal for monitoring growth for a longer period, there was a clinical complication that led to death. However, in the first 6 months after surgery, we were able to observe growth of at least 0.3 cm in the transposed fibula segment, which indicates that the growth potential had been preserved.
CONCLUSIONS:
The extensible internal fixation device stabilizes the reconstruction and allows for growth, through the sliding mechanism between the plates. The proximal fibular segment, transferred using this technique, preserves its blood supply and the growth function of the transplanted physis. We consider that this technique, of transferring the fibula with its growth cartilage, can be used in the reconstruction of injuries that affect the proximal metadiaphyseal segment of the tibia in children, where the tibial epiphysis can be preserved.
Bibliographic references
1. Digby KH. The measurement of diaphysial growth in proximal and distal directions. J Anat Physiol 1916 Jan;50(Pt 2):187-8.
2. Mercuri M, Capanna R, Manfrini M et al. The management of malignant bone tumors in children and adolescents. Clin Orthop Related Res 1991; 264:156-68.
3. Boyer MI, Bray PW, Bowen CVA. Epiphyseal plate transplantation: an historical review. Br J Plast Surg 1994; 47:563-9.
4. Saghieg S, Abboud MR, Muwakkit SA, Saab R, Haidar R. Seven-year experience of using Rephiphysis expandable prosthesis in children with bone tumors. Pediatric Blood Cancer. 2010; 55(3): 457-63.
5. Gosheger G, Gebert C, Ahrens H, Streitbuerger A, Winkelmann W, Hardes J. Endoprosthetic reconstruction in 250 patients with sarcoma. Clin Orthop Relat Res 2006; 450:164-71.
6. Campanacci L, Manfrini M, Colangeli M, Ali N, Mercury M. Long-term results in children with massive bone osteoarticular allografts of the knee for high-grade osteosarcoma. J Pediatr Orthop 2010; 30(8): 919-27.
7. Weitao Y, Qiqing C, Sogtao G, Jiaqiang W. Epiphysis preserving operations for the treatment of lower limb malignant bone tumors. Eur J Surg Oncol 2012: 38(12)1165-70.
8. Fang B, Yi C, Zhang Q, Li Y, Wei Q, He W Zeng Z. Combined epiphyseal preservation and autograft bone transfer in treatment of childhood osteosarcoma. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2013: 27(1): 45-9.
9. Davis A G. Fibular replacement for tibial defects. J Bone Joint Surg 1944; Vol XXVI. AT THE. 2. April.
10. Agiza ARH. Treatment of tibial osteomyelitic defects and infected pseudoarthroses by the Huntigton fibular transfer operation. J Bone Joint Surg 1981; 63-A(5):814-9.
11. Langenskiöld A. Hahn’s operation for pseudoarthrosis after osteomyelitis of the tibia in children: A report of three cases. Acta Orthop Scand 1983; 54:714-20.
12. Date AS, Solanki SB, Badhe NP, Sonsale PD, Pandit HG. Management of gap non-union of tibia by tibialisation of ipsilateral vascular fibula. J Postgrad Med 1996; 42(4):109-11.
13. Taylor GI, Miller GDH, Ham FJ. The free vascularized bone graft: A clinical extension of microvascular techniques. Plast Reconstr Surg 1975; 55(5):533-44.
14. Pho RWH, Patterson MH, Kour AK, Kumar VP. Free vascularized epiphyseal transplantation in upper extremity reconstruction. J Hand Surg 1988; 13B:440-7.
15. Baptista PPR, Yonamine ES. Device for extensible internal fixation. Rev Bras Ortop 2001; 36(7): 273-8.
16. Justolin LT, Rahal SC, Baptista PPR, Yonamine MJ, Mamprim J, Balieiro CC. Use of extensible internal device in the femur of young dogs. Veterinary and Comparative Orthopedics and Traumatology 2008 21 2: 133-139.
17. Holzapfel BM, Pilge H, Toepfer A, Jakubietz RG, Gollwitzer H, Rechl H, von Eisenhart-Rothe R, Rudert M. Proximal tibial replacement and alloplastic reconstruction of the extensor mechanism after bone tumor resection. Oper Ortuop Traumatol 2012; 24(3):247-62.
18. Jeys LM, Kulkami A, Grimer RJ, Carter SR, Tilman RM, Abudu A. Endoprosthetic reconstruction for the treatment of musculoskeletal tumors of the appendicular skeleton and pelvis. J Bone Joint Surg Am 2008: 90(6): 1265-71.
19. Wang TY, Dormand JP, Chang B. Soft-tissue optimization of limb salvage with knee endoprosthesis: 10 year experience at the Children’s Hospital of Philadelphia. Ann Plast Surg, 2012: 69(5): 560-64.
20. Honoki K, Kobata Y, Miyauchi Y et al. Epiphyseal preservation and an intercalary vascularized fibular graft with hydroxypatite composites: Reconstruction in metaphyseal osteosarcoma of the proximal tibia: A case report. Arch Orthop Trauma Surg 2008; 128(2):189-93.
21. Muscolo DL, Ayerza MA, Aponte-Tinao LA, Ranalletta M. Partial epiphyseal preservation and intercalary allograft reconstruction in high-grade metaphyseal osteosarcoma of the knee. J Bone Joint Surg Am 2004 Dec;86-A(12):2686-93.
22. Hriscu M, Mojallal A, Breton P, Bouletreau P, Carret JP. Limb salvage in proximal humerus malignant tumors: The place of free vascularized fibular graft. J Reconstr Microsurg 2006; 22(6):415-21.
23. Kapukaya A, Subasi M, Kandiya E, Ozates M, Ylmaz F. Limb reconstruction with the callus distraction method after bone tumor resection. Arch Orthop Trauma Surg. 2000;120(3-4): 215-8.
24. Baptista PPR, Guedes A, Reggiani R, Lavieri R, Pires CEF. Fibula tibialization: Description of surgical approach. Rev Bras Ortop 1998; 33(11):861-6.
25. Campbell WC. Transference of the fibula as an adjunct to free bone graft deficiency: Report of three cases. J Orthop Surg 1919; 1:625.
26. Carnesale PL, Guerrieri AG. Fibular transplant for loss of substance of tibia. J Bone Joint Surg 1955; 37A(1):204-6.
27. Chung YK, Chung S. Ipsilateral island fibula transfer for segmental tibial defects: Antegrade and retrograde fashion. Plast Reconstr Surg 1998; 101(2):375-82.
28.Shapiro MS, Endrizzi DP, Cannon RM, Dick HM. Treatment of tibial defects and nonunions using ipsilateral vascularized fibular transposition. Clin Orthop Related Res 1993; 296:207-12.
29. Weiland AJ, Moore JR, Daniel RK. Vascularized bone autografts: Experience with 41 cases. Clin Orthop Related Res 1983; 174:87-95.
30. Arai K, Toh S, Tsubo K, Nishikawa S, Narita S, Miura H. Complications of vascularized fibula graft for reconstruction of long bones. Plast Reconstr Surg 2002; 109:2301-6.
31. Tsai TM, Ludwig L, Tonkin M. Vascularized fibular epiphyseal transfer. Clin Orthoped 1986; 210:228-34.
32. Helferich U. Versuche über die Transplantation des Intermediarknorpels wachsender Rohrenknochen. Deutsche Z Chir 1899; 51:564-73.
33. Heller E. Experimentelle Utersuchungen über die Transplantation des Intermediarknorpels in Form der halbseitigen Gelenktransplantation. Arch Klin Chir 1914; 104:843-954.
34. Haas SL. Further observations on the transplatation of the epiphyseal cartilage plate. Surg Gynecol Obstet 1931; 52:958-63.
35. Straub GF. Anatomic survival, growth and physiological function of an epiphyseal bone transplant. Surg Gynecol Obstet 1929; 48:687-90.
36. Sawaizumi M, Maruyama Y, Okajima K, Motegi M. Free vascularized ephiphyseal transfer designed on the reverse anterior tibial artery. British Journal of Plastic Surgery(1991, 44, 57-59.
37. Concannon MJ, Croll GH, Boschert MK, Gaines RW, Puckett CL. Free fibular transfer in a growing individual (long-term results). Microsurgery 1993; 14:624-7.
38. Becker LM, Zuker RM. Vascularized fibular epiphyseal transplantation for limb salvage following bone tumor excision. Can J Plast Surg 1999; 7(2):65-73.
39. Menezes-Leite MC, Dautel G, Duteille F, Lascombes P. Transplantation of the proximal fibula based on the anterior tibial artery: Anatomical study and clinical application. Surg Radiol Anat 2000; 22:235-8.
Authors of the case
Author: Prof. Dr. Pedro Péricles Ribeiro Baptista
Orthopedic Oncosurgery at the Dr. Arnaldo Vieira de Carvalho Cancer Institute
Office : Rua General Jardim, 846 – Cj 41 – Cep: 01223-010 Higienópolis São Paulo – SP
Phone: +55 11 3231-4638 Cell:+55 11 99863-5577 Email: drpprb@gmail.com