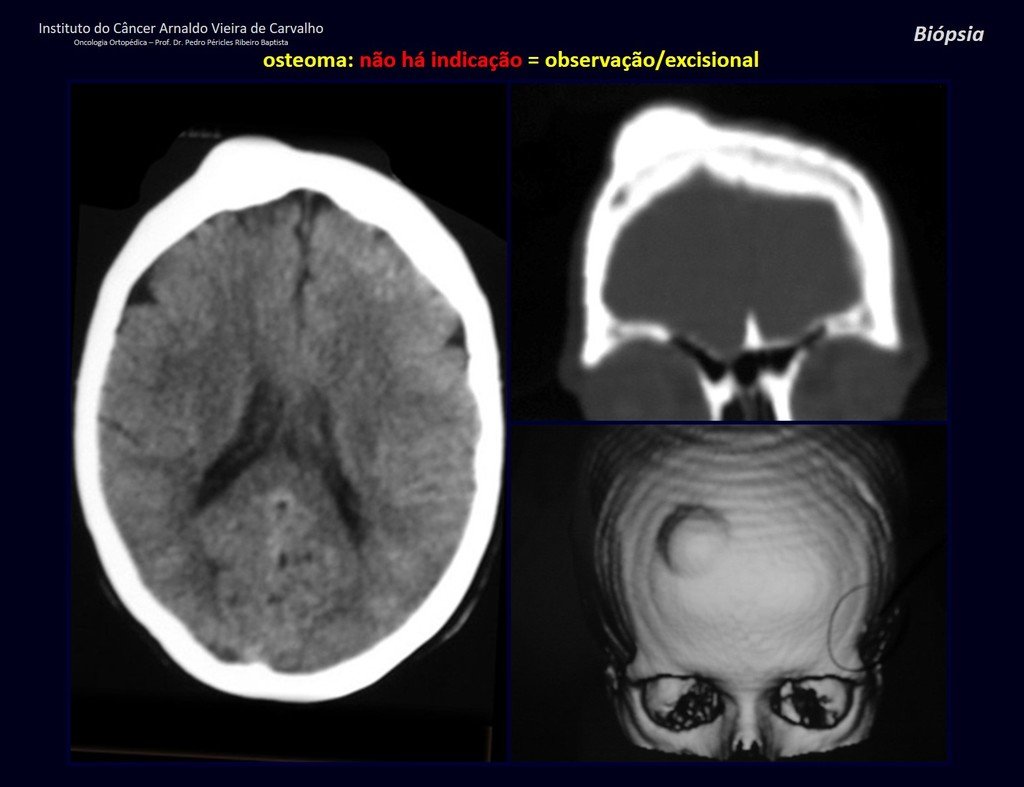
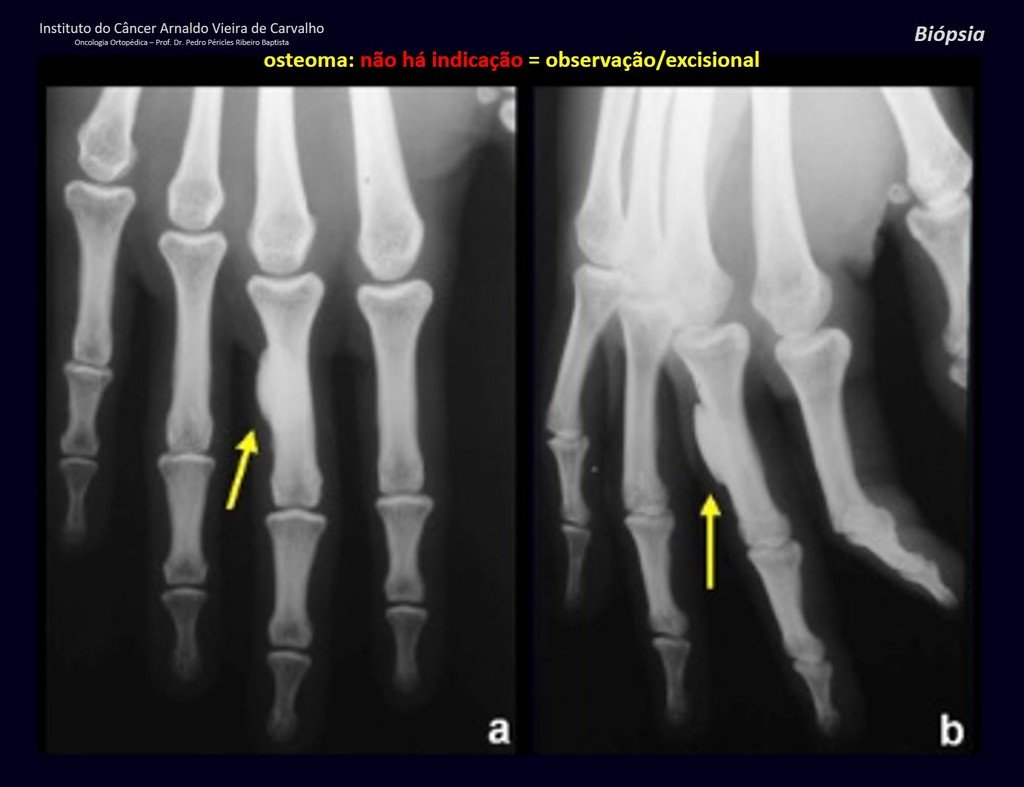
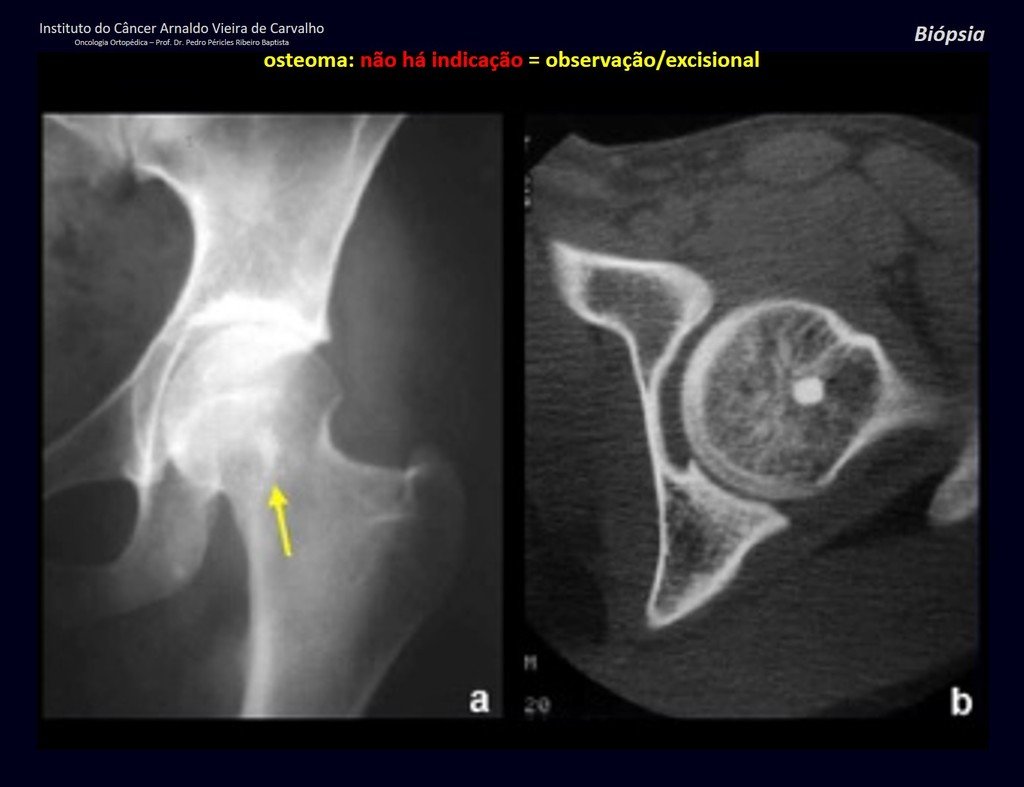
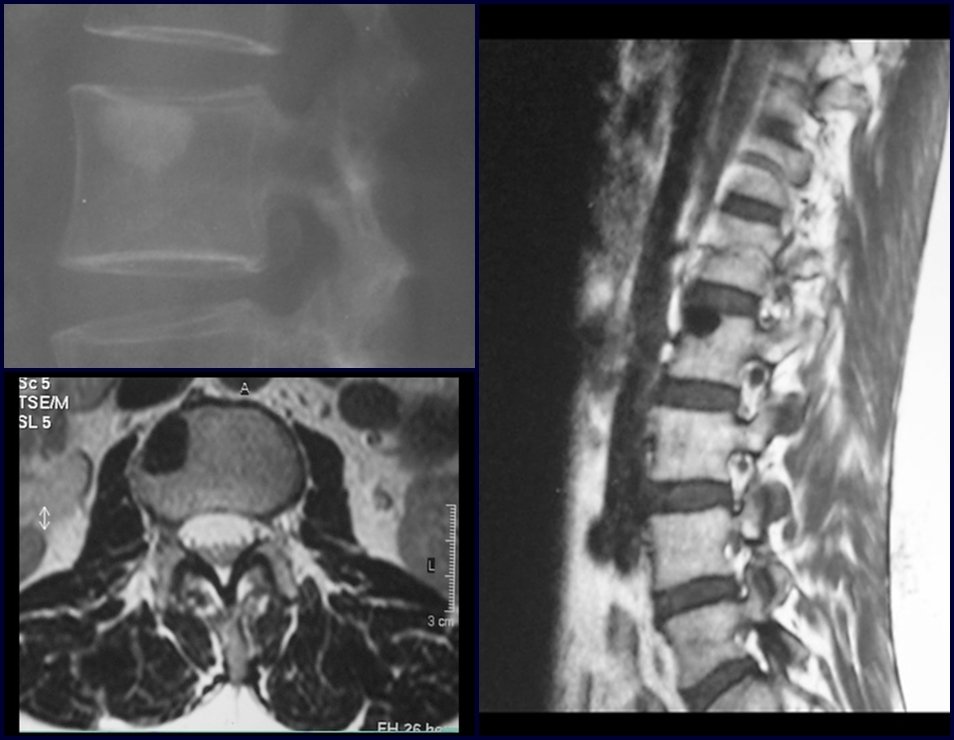
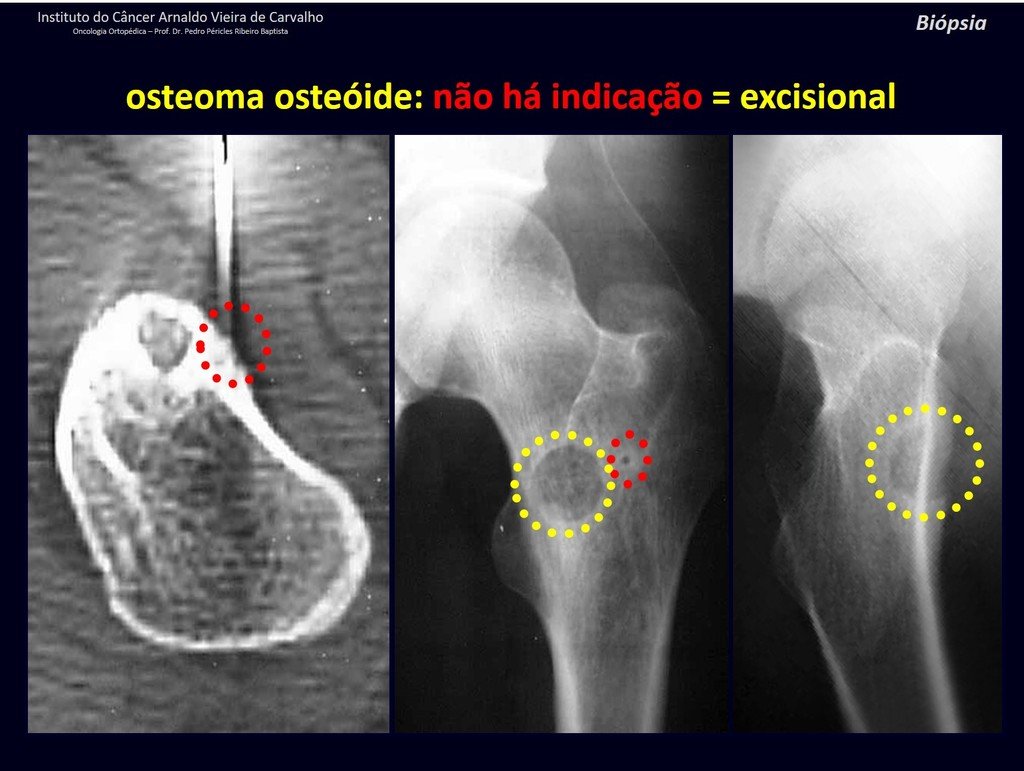
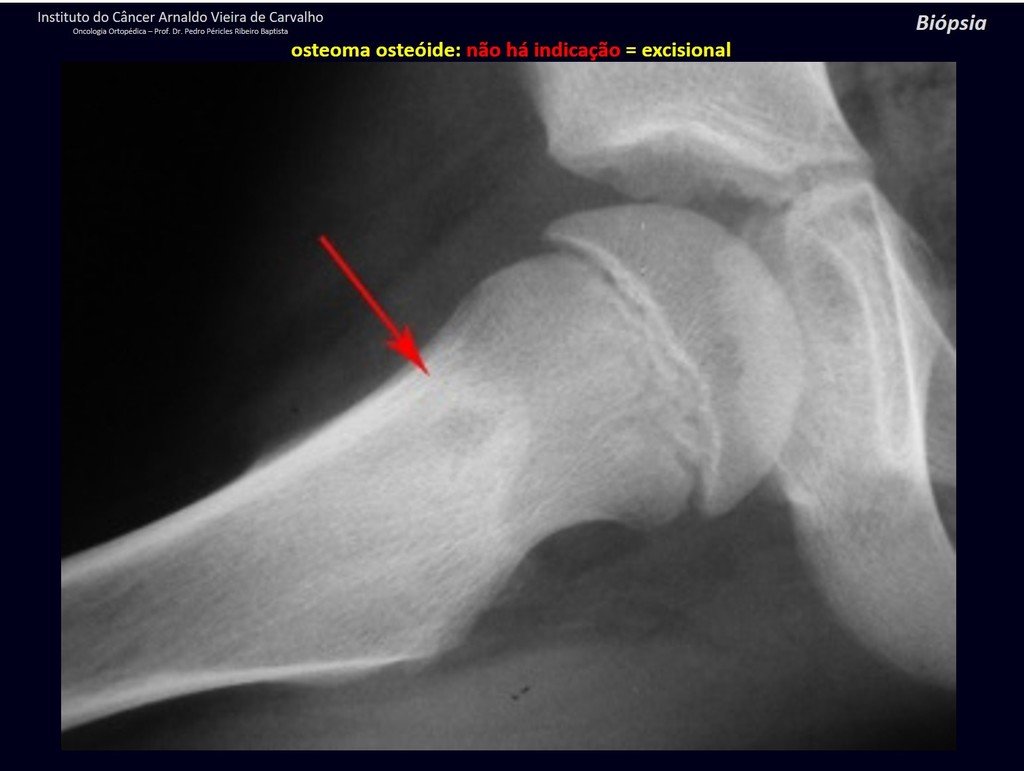
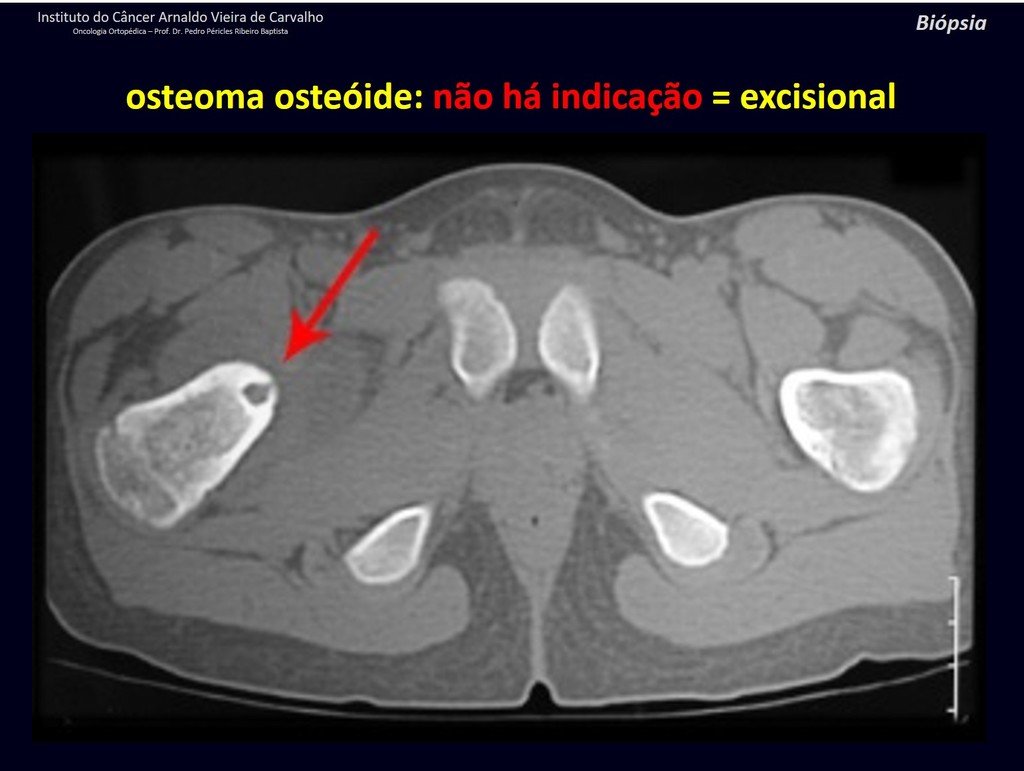
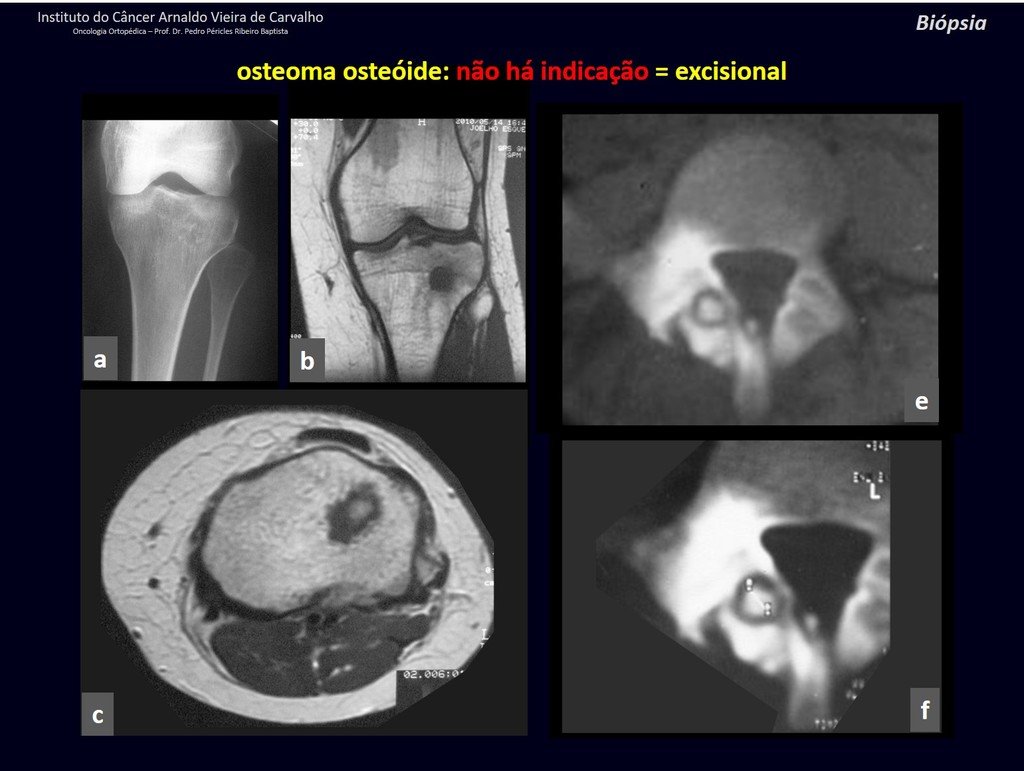
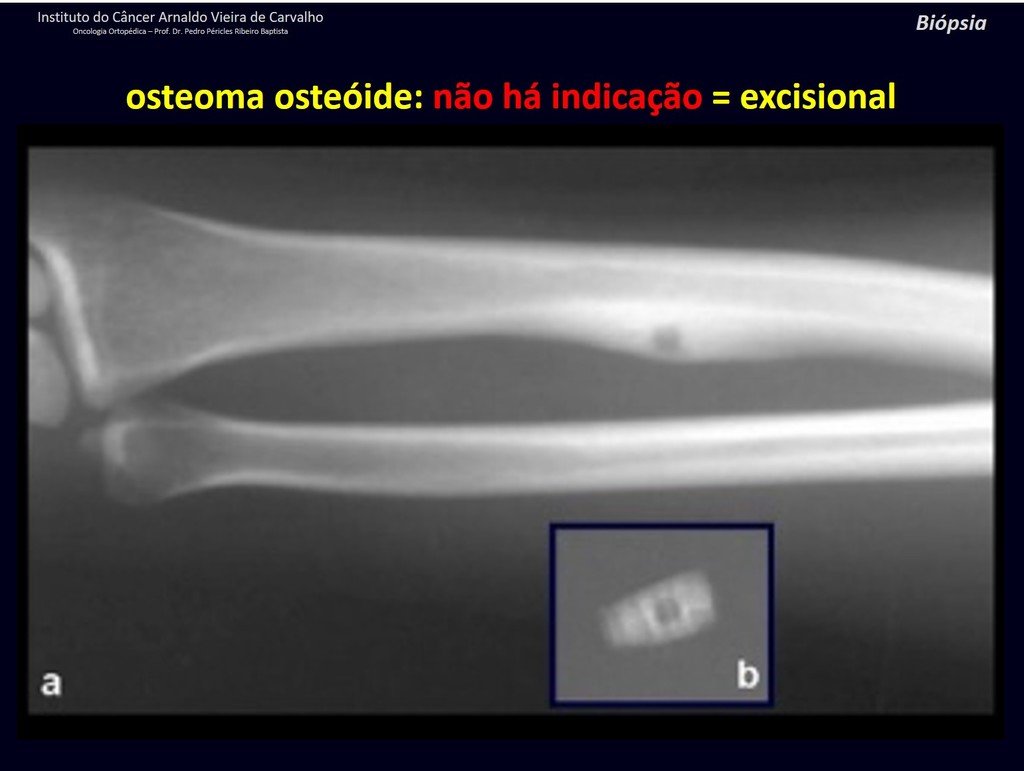
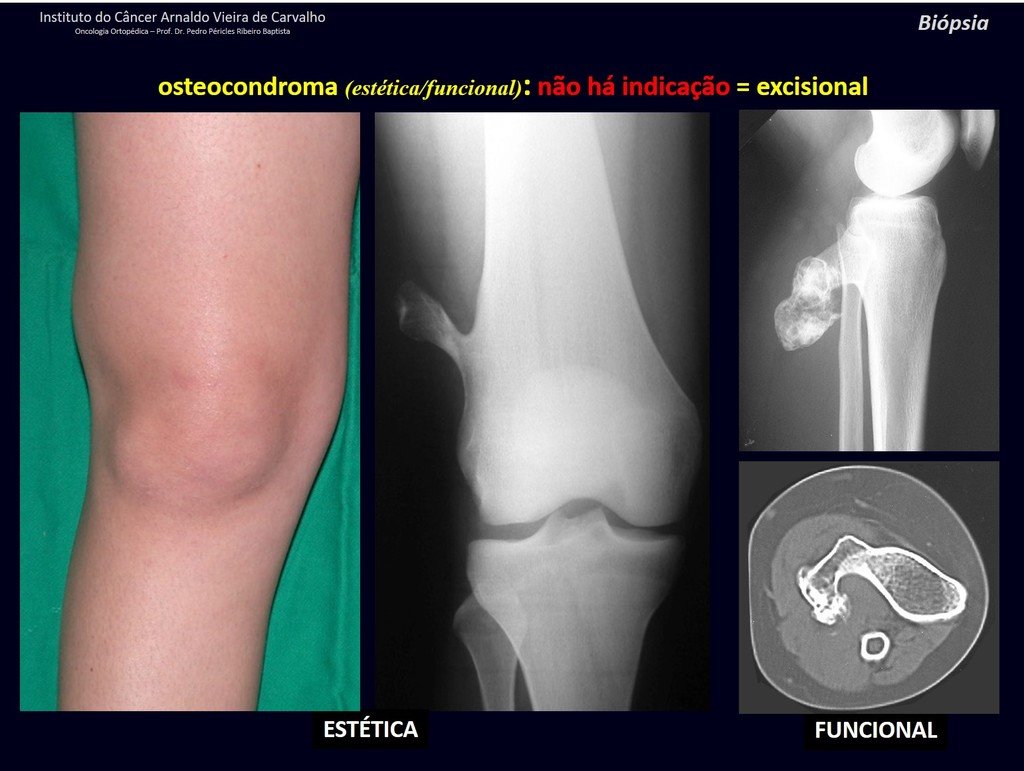
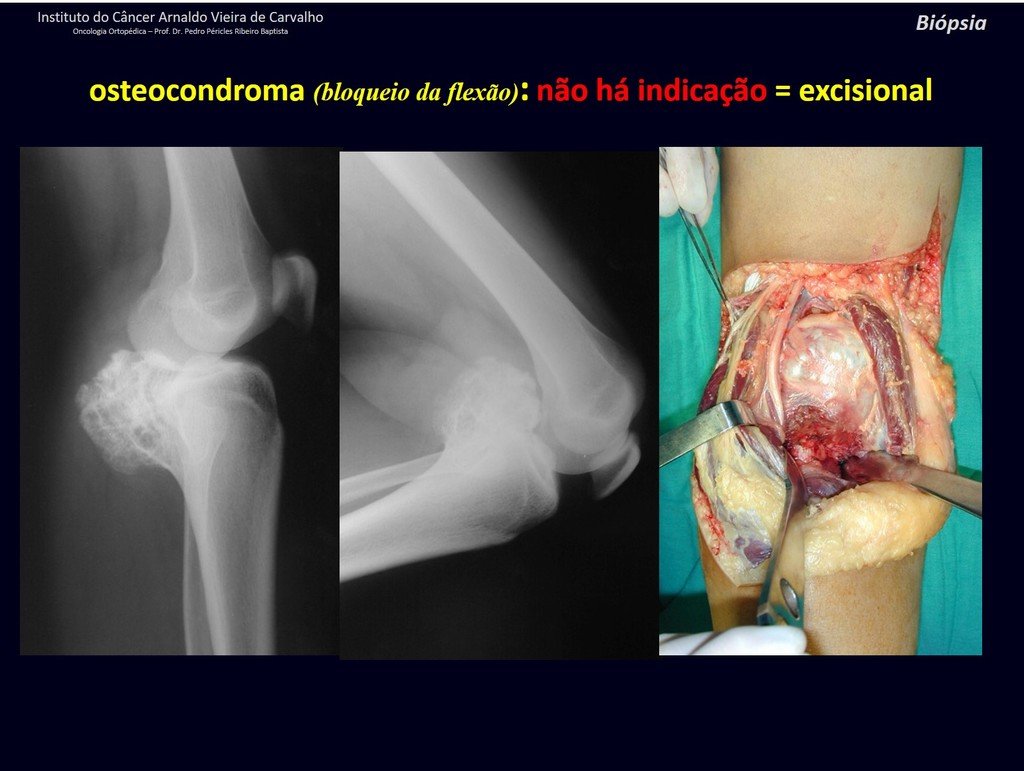
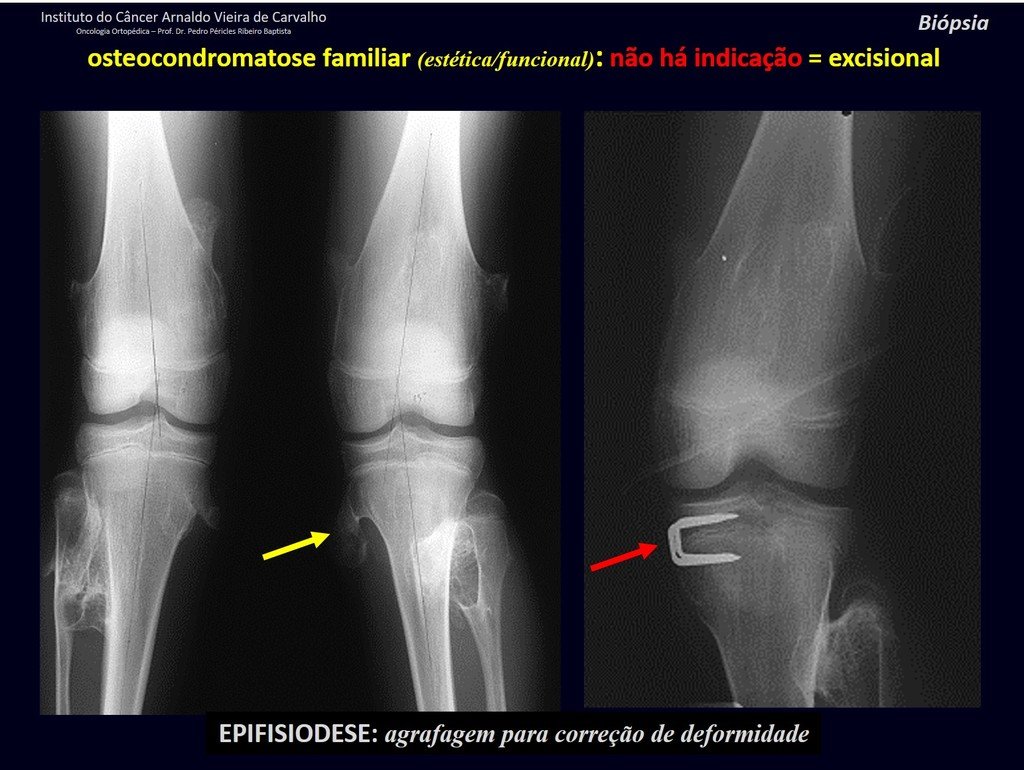
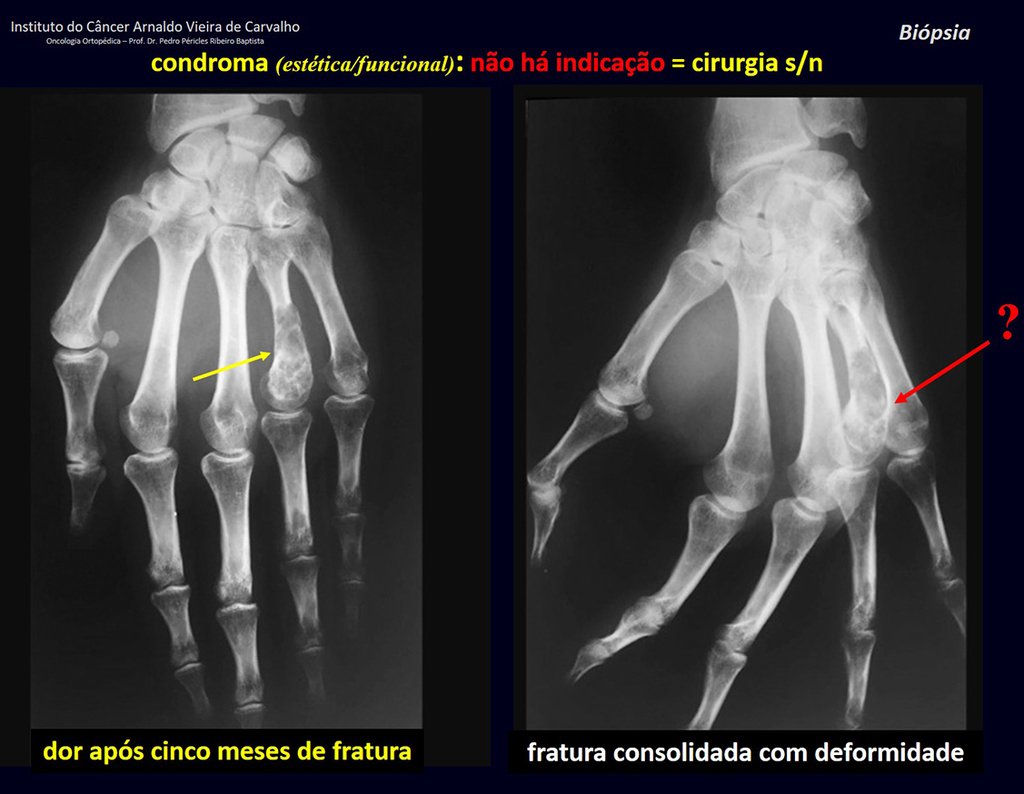
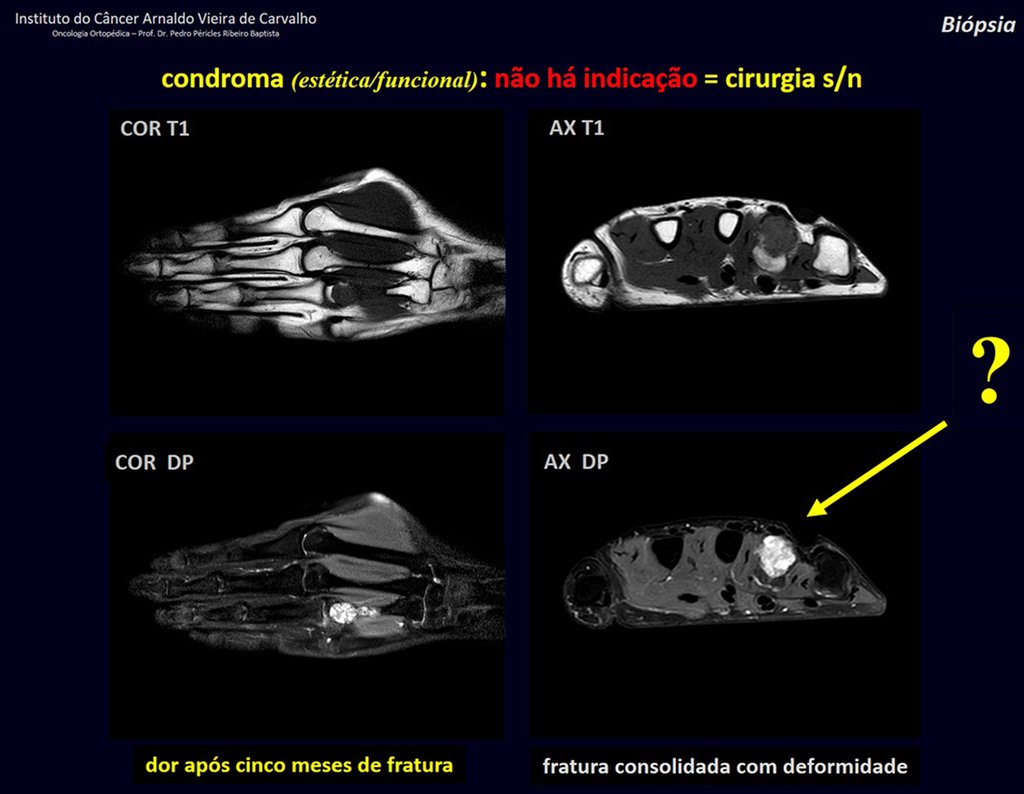
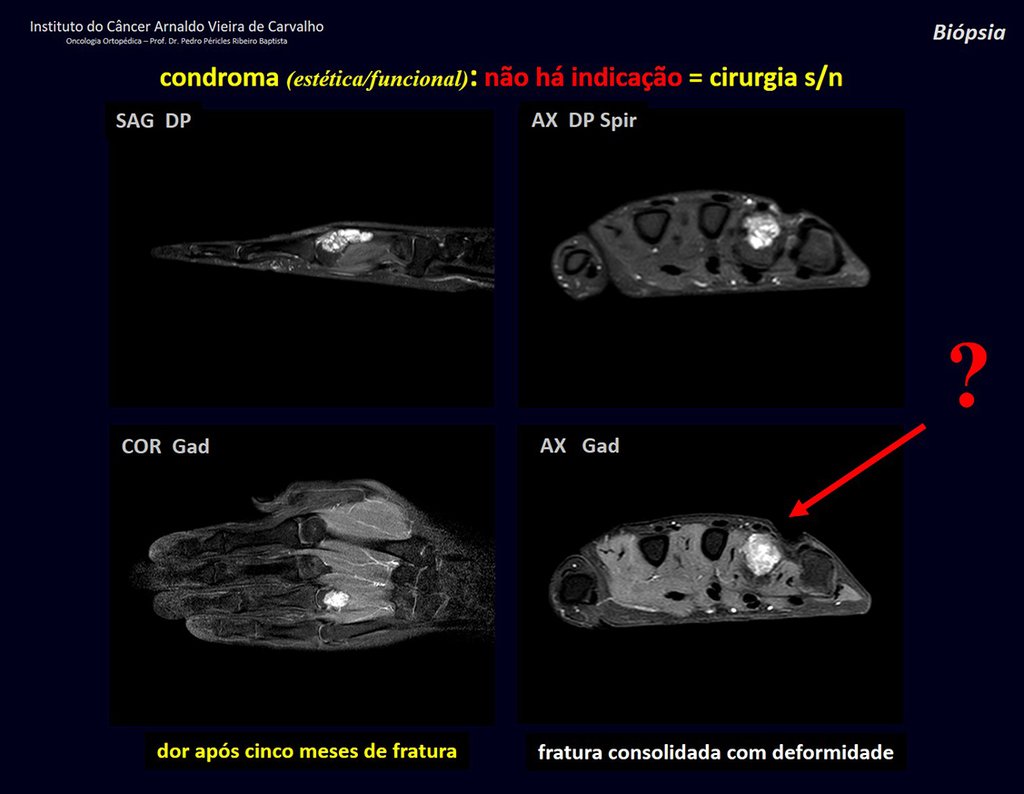
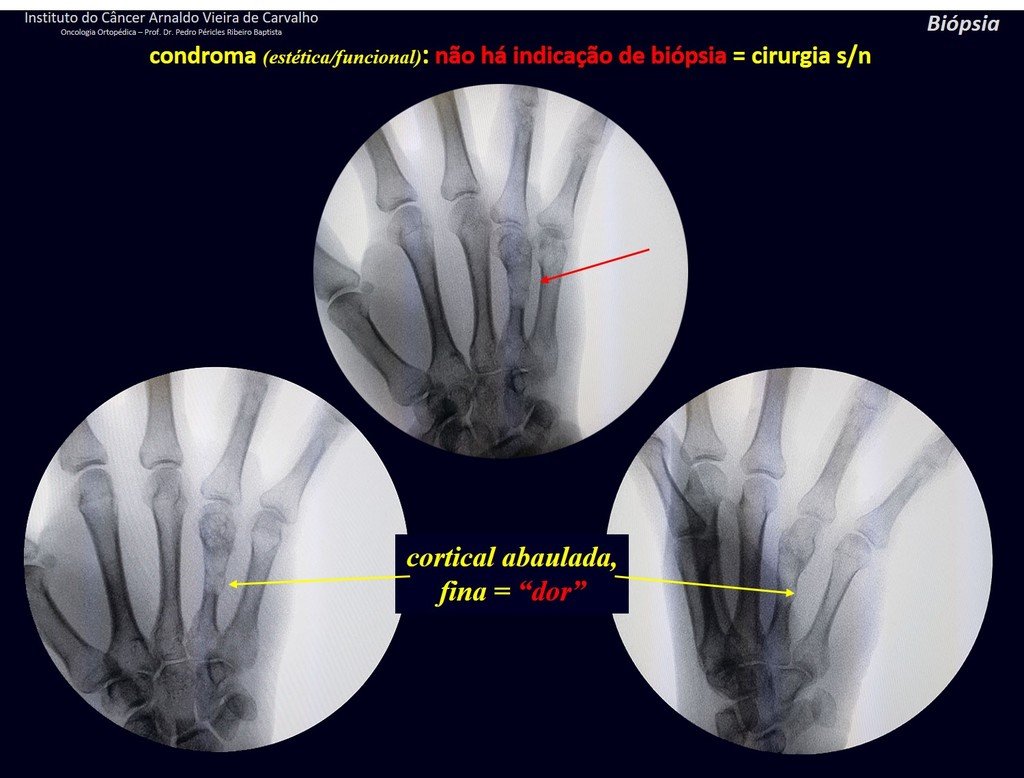
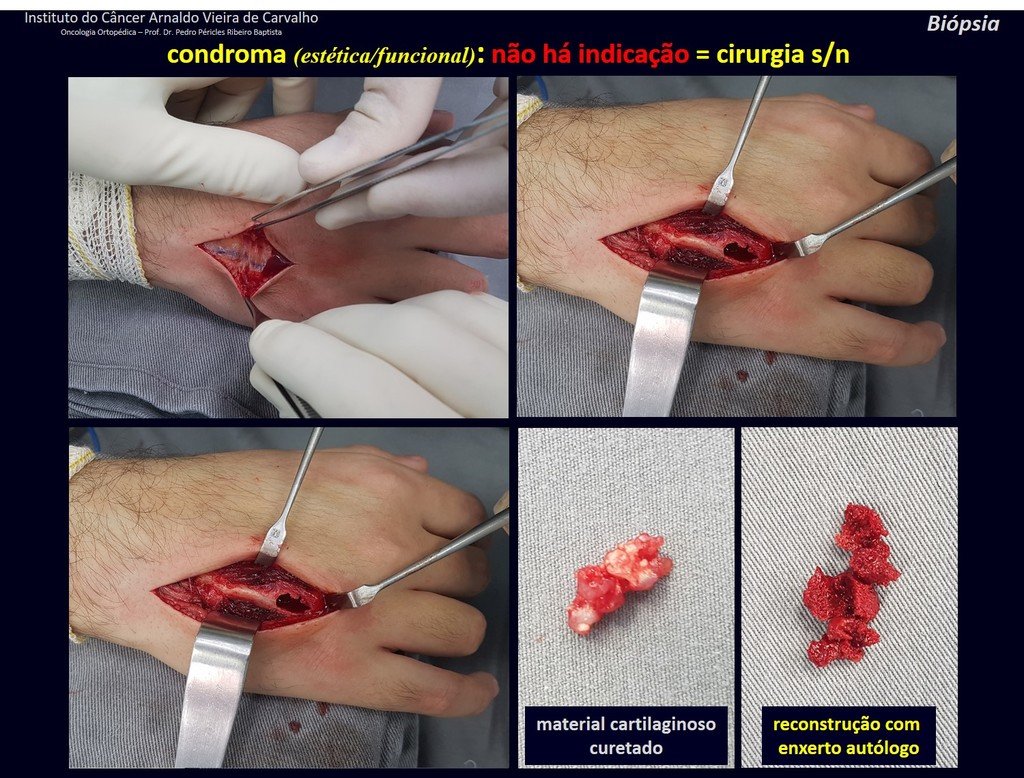
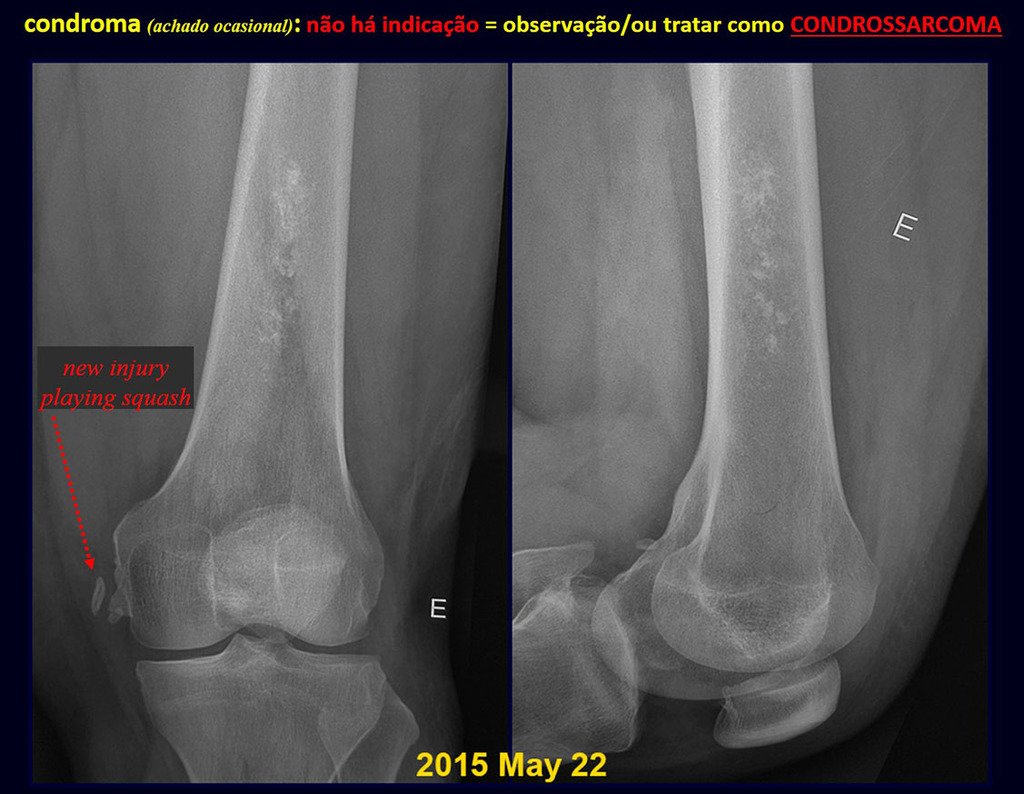
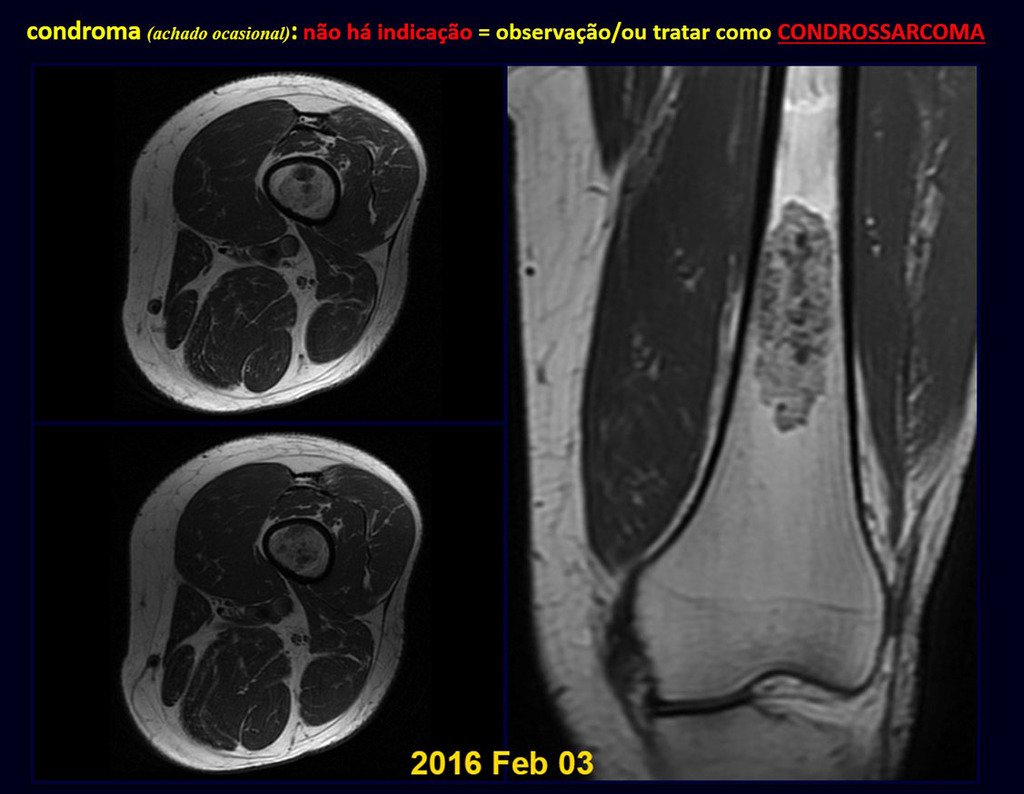
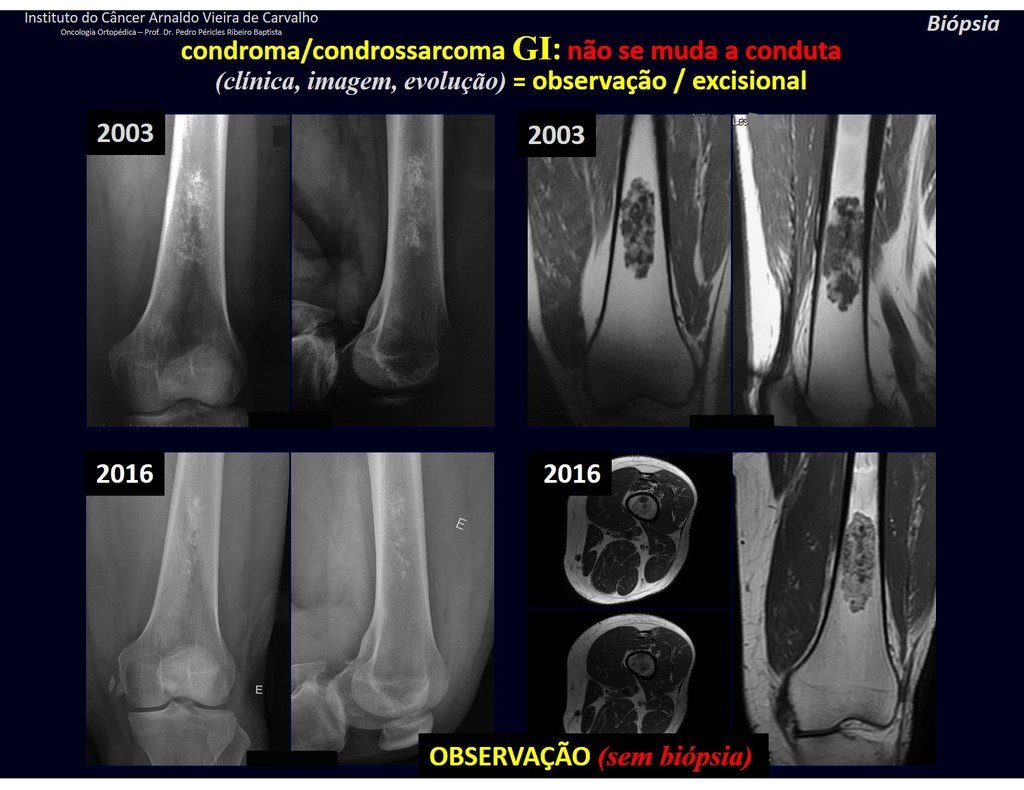
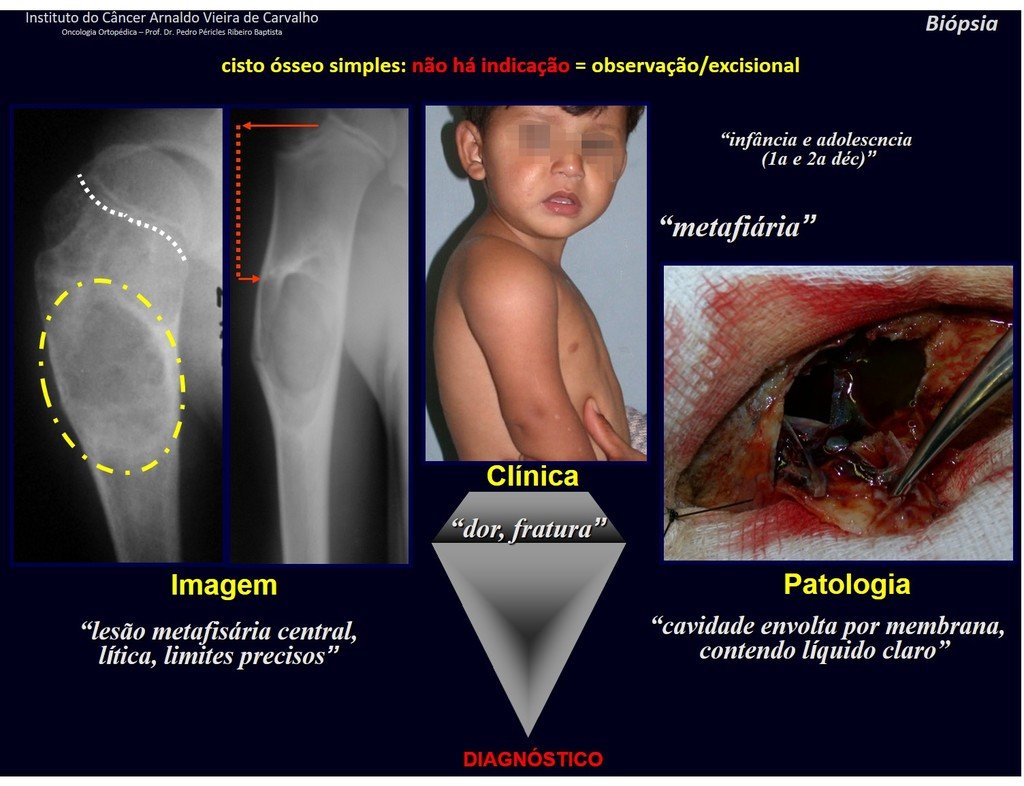
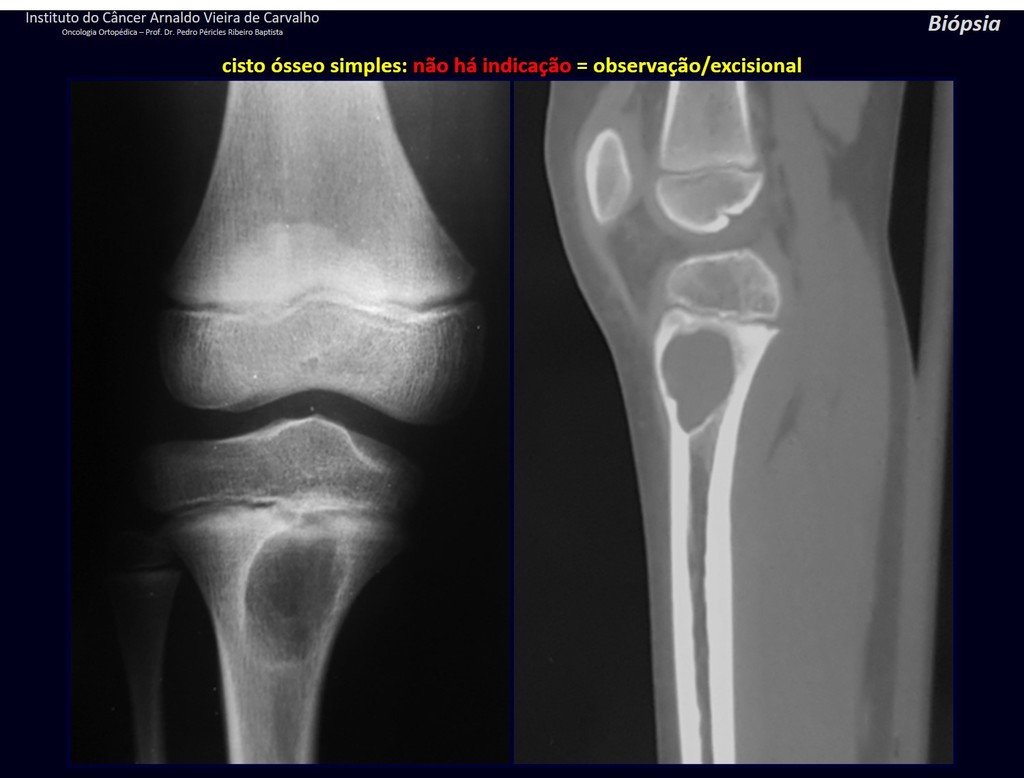
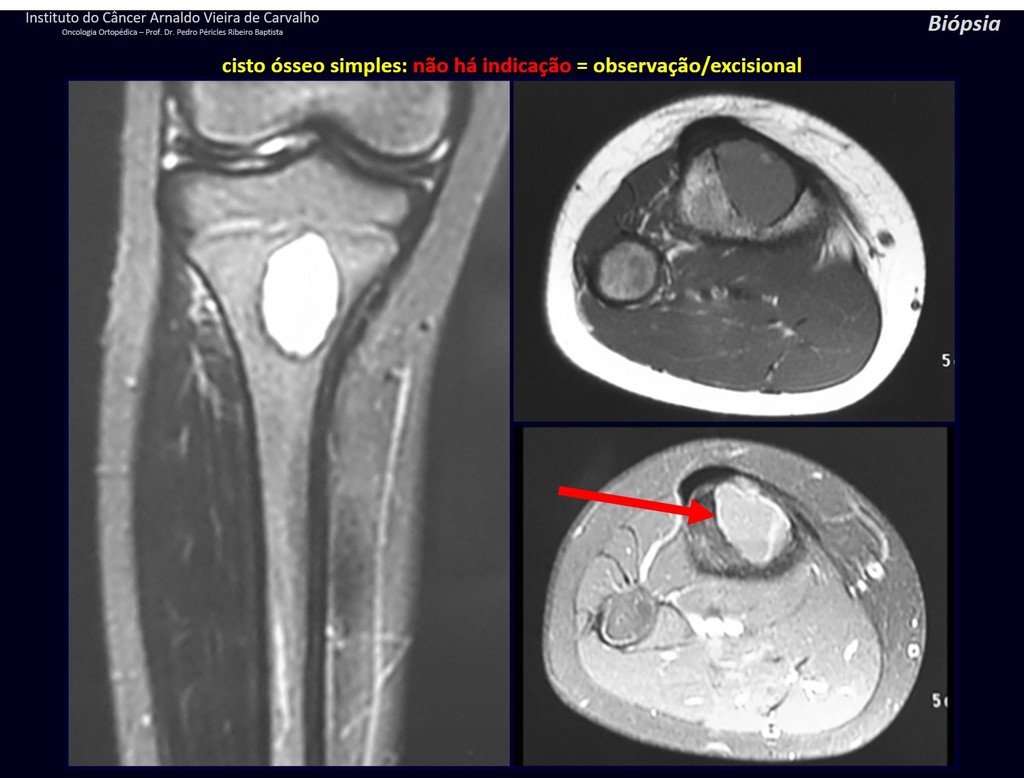
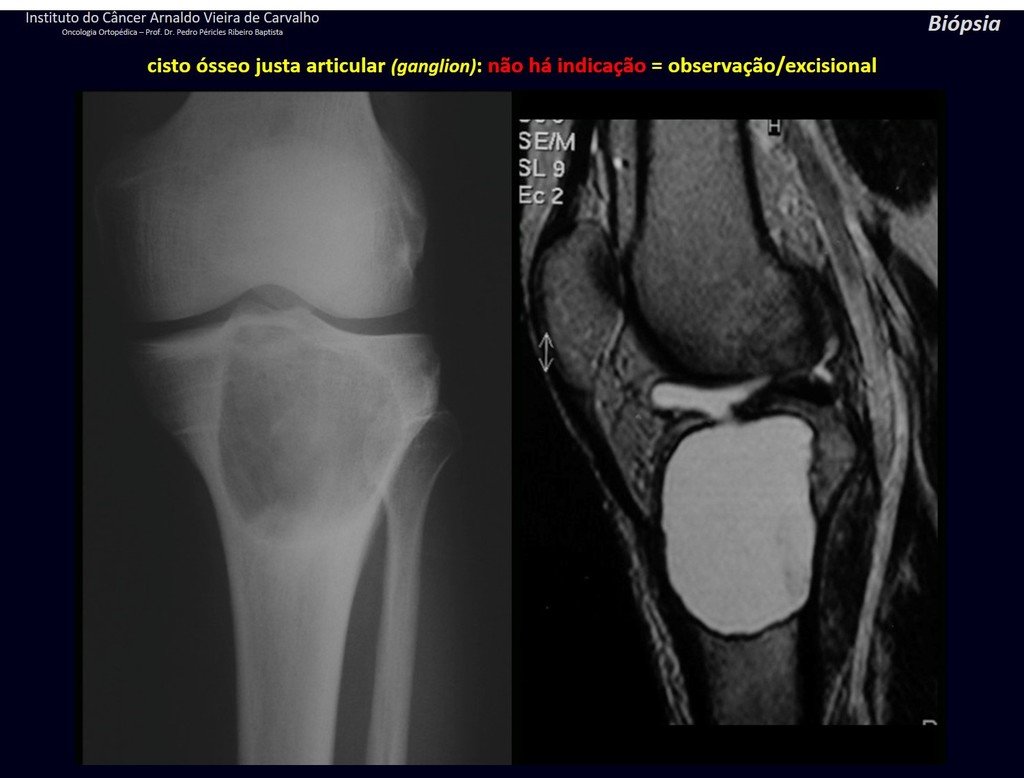
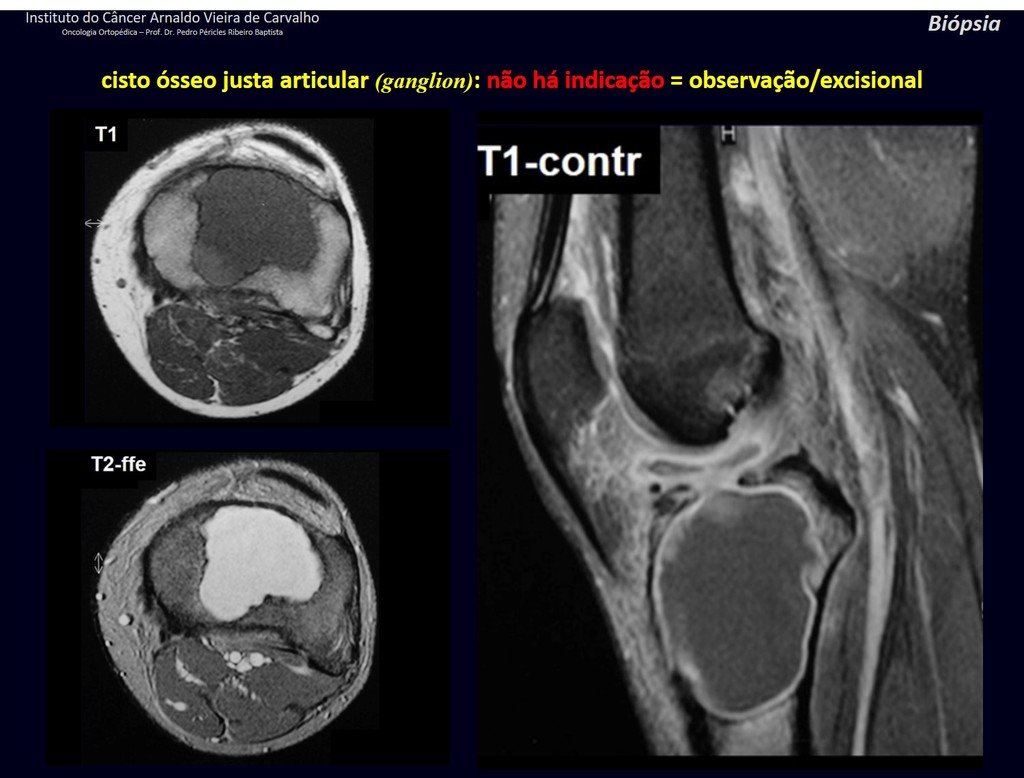
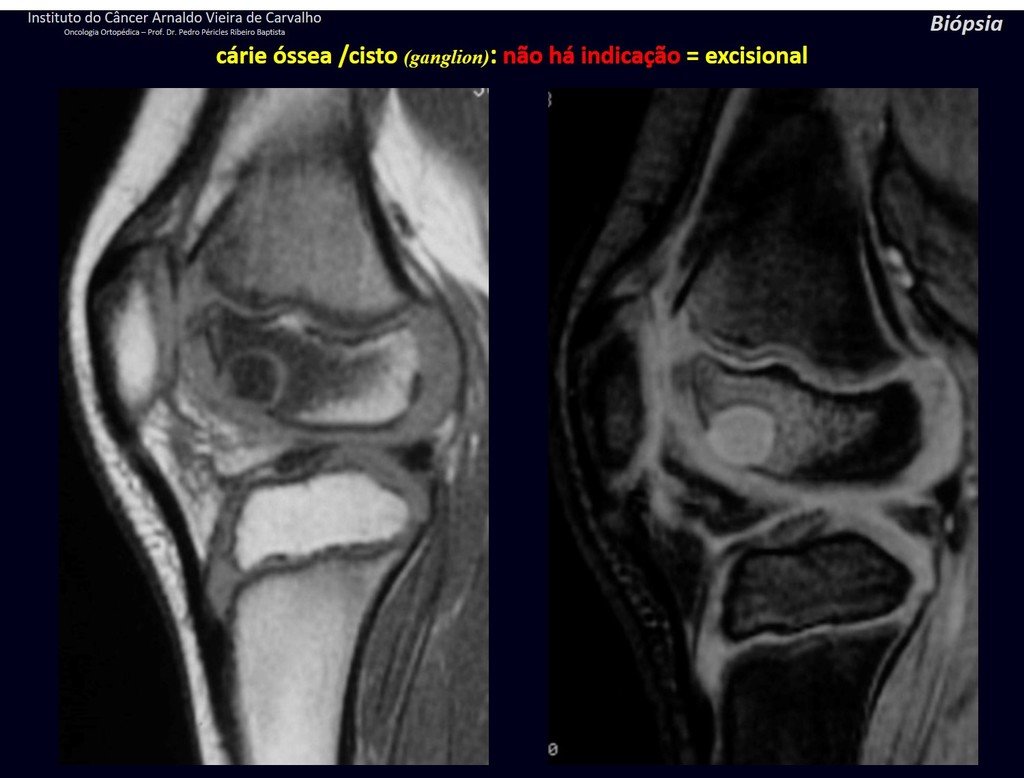
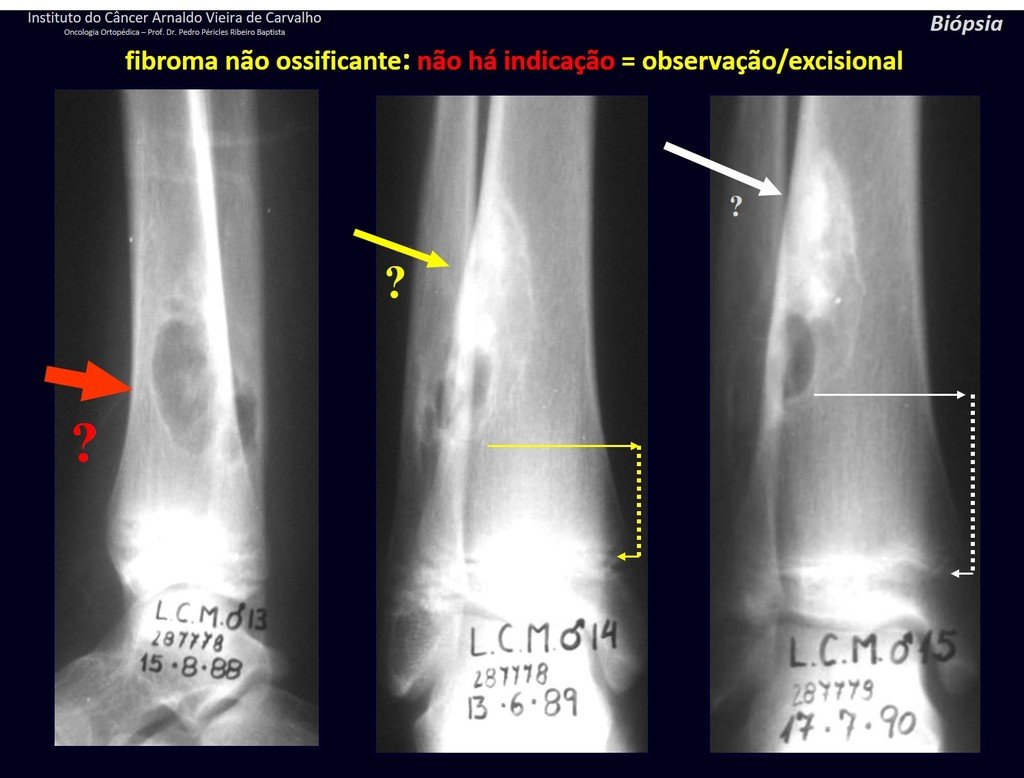
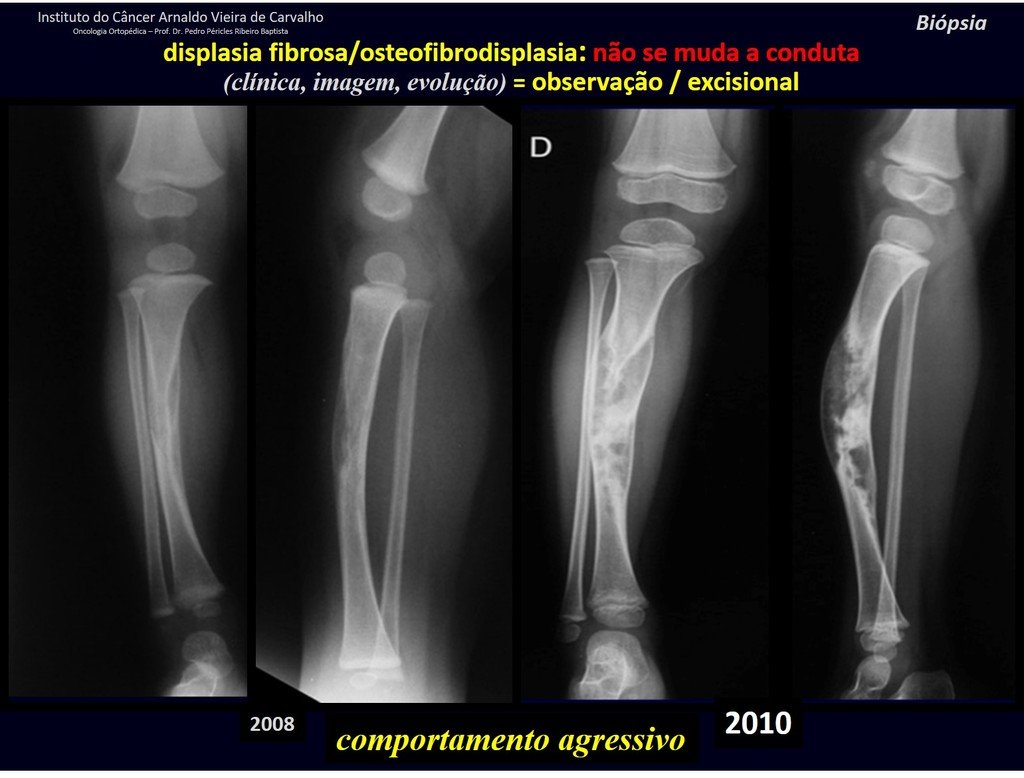
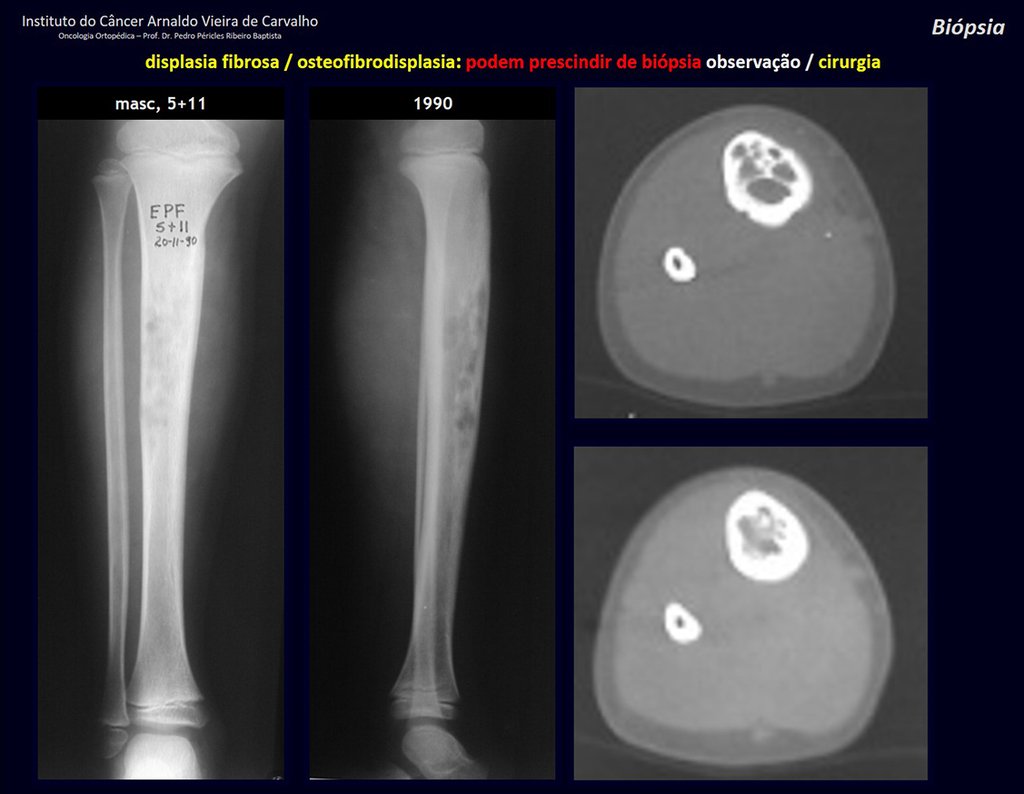
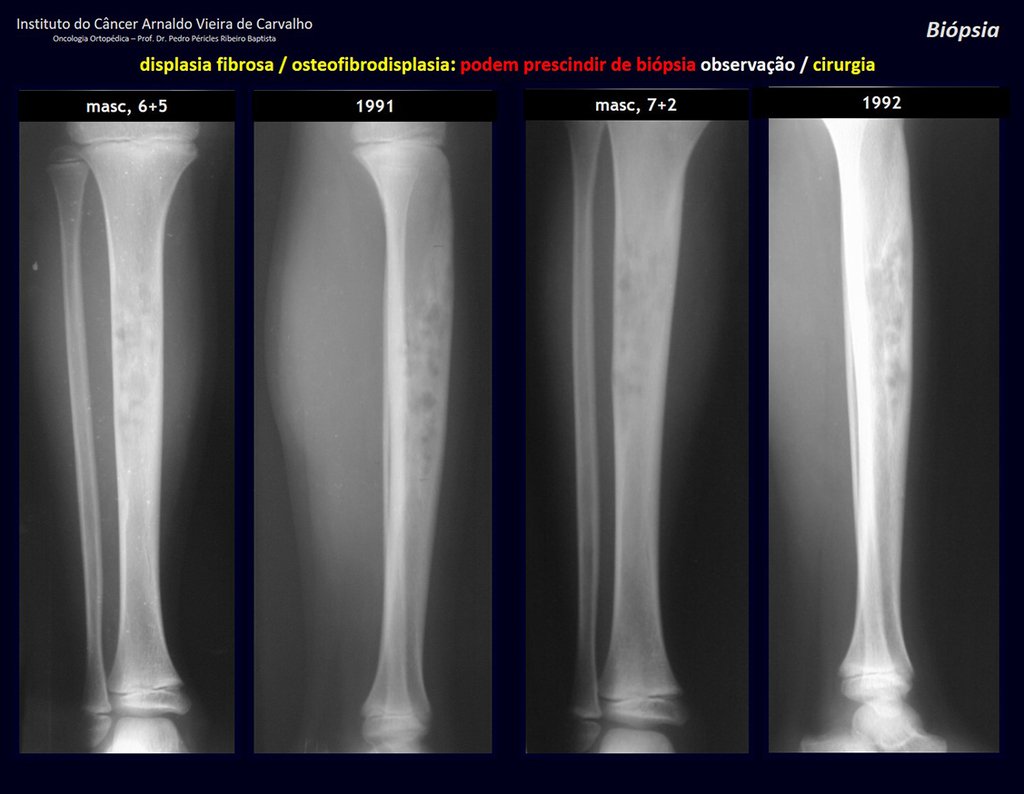
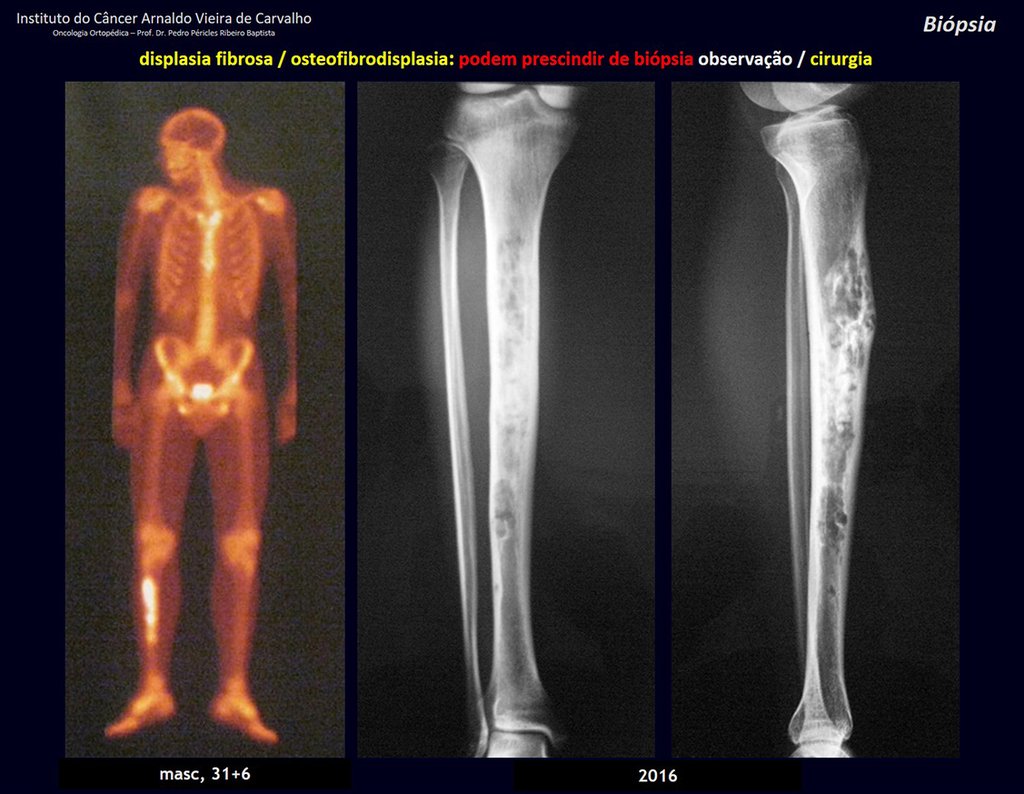
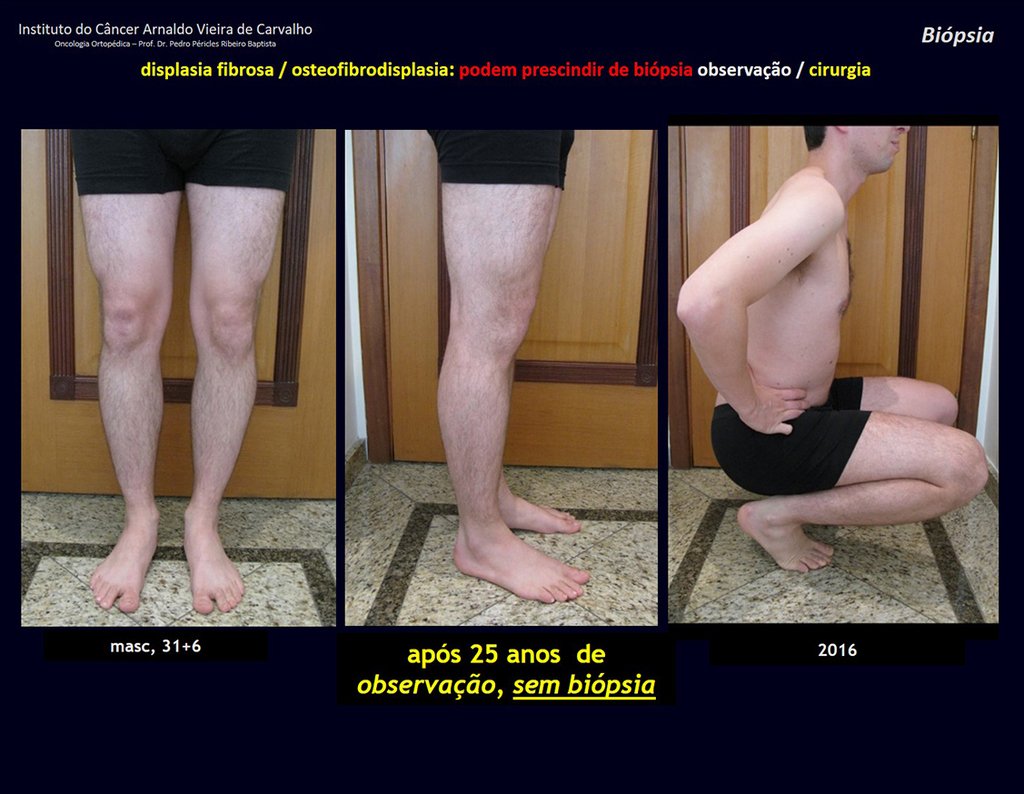
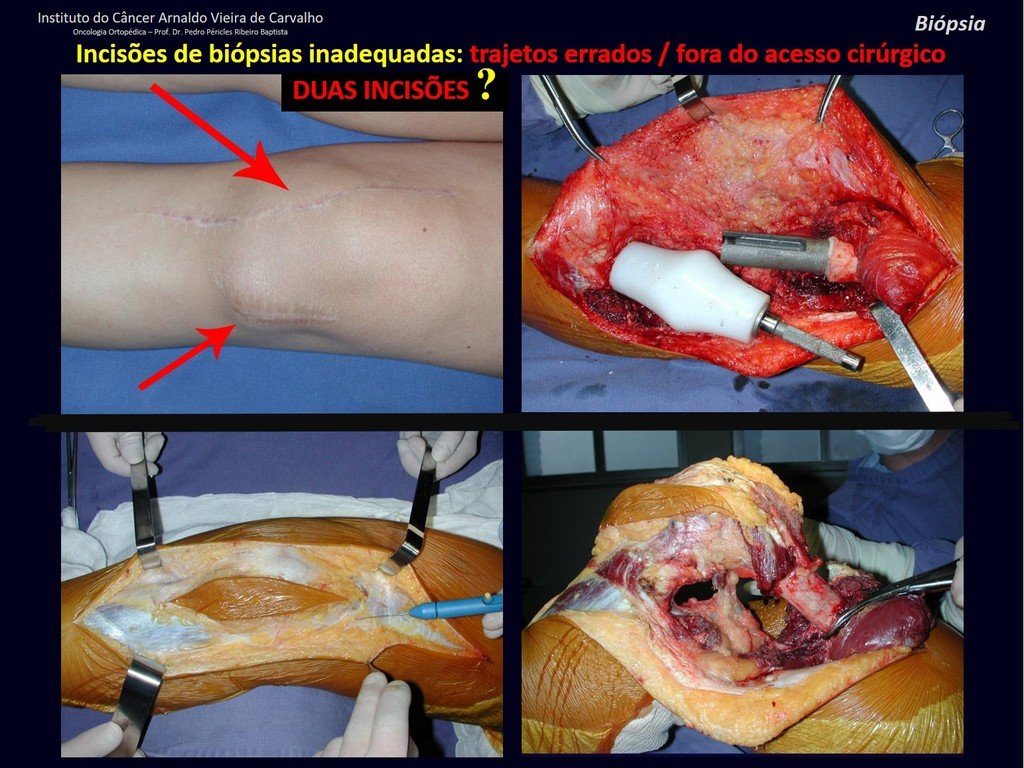
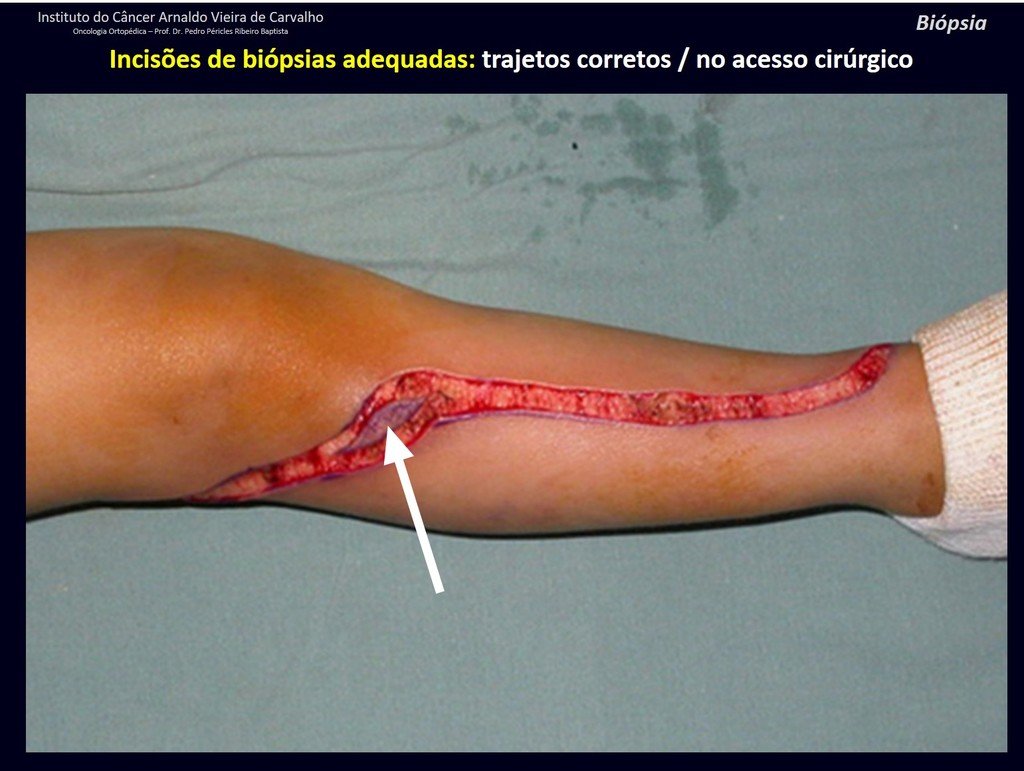
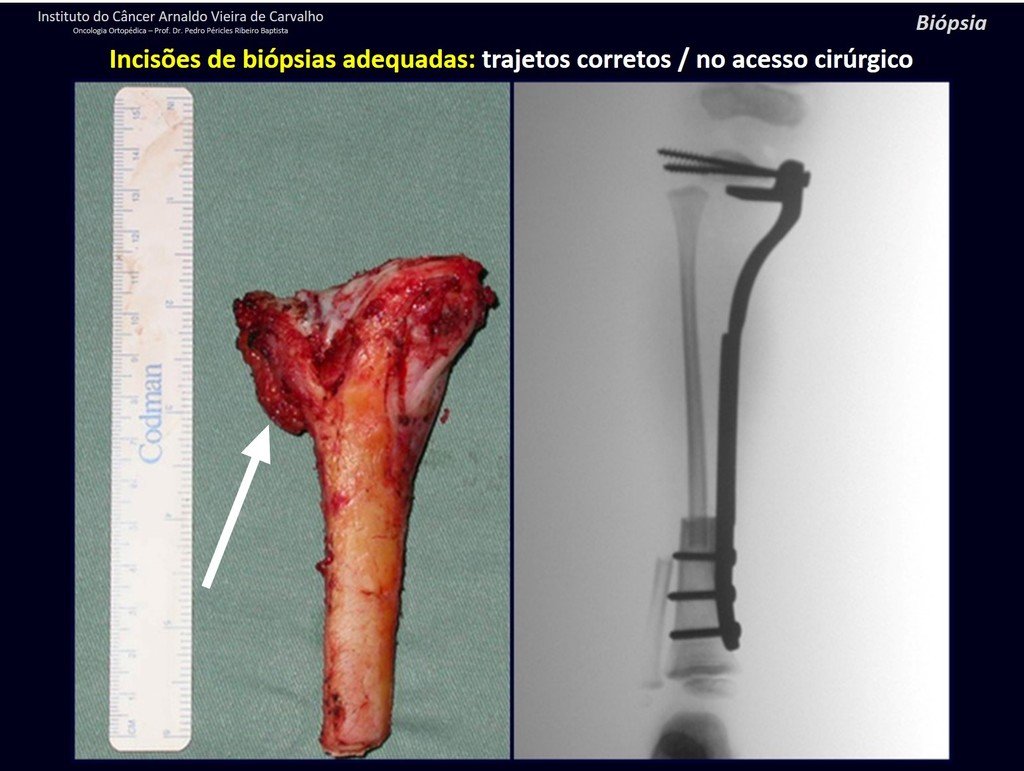
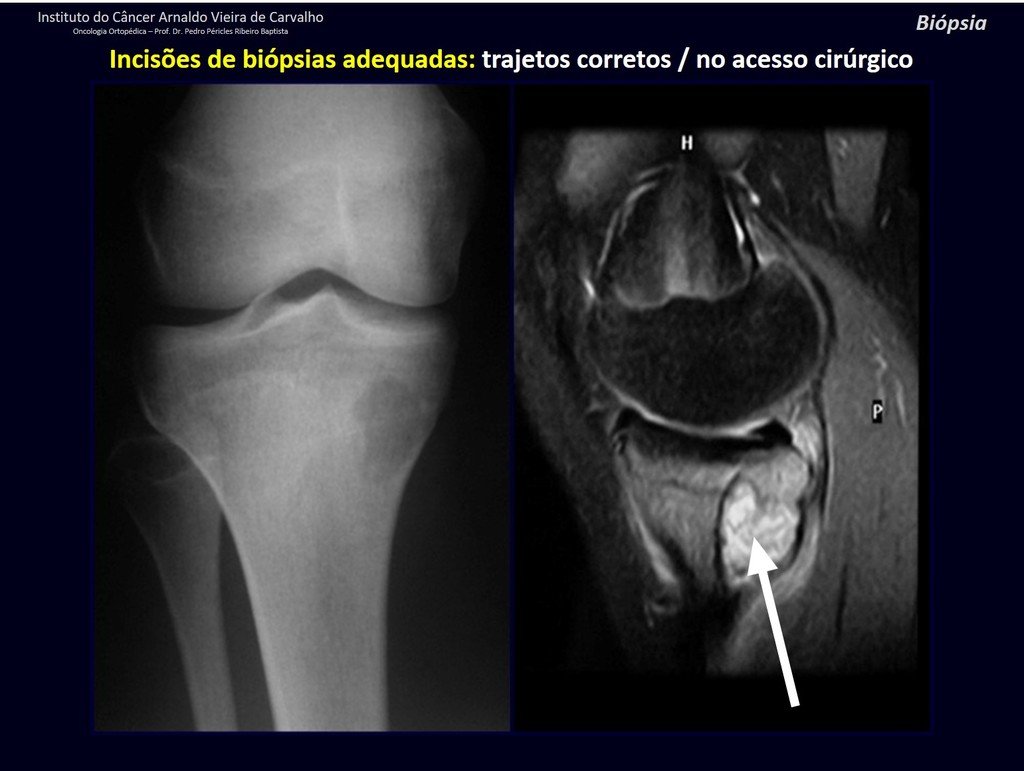
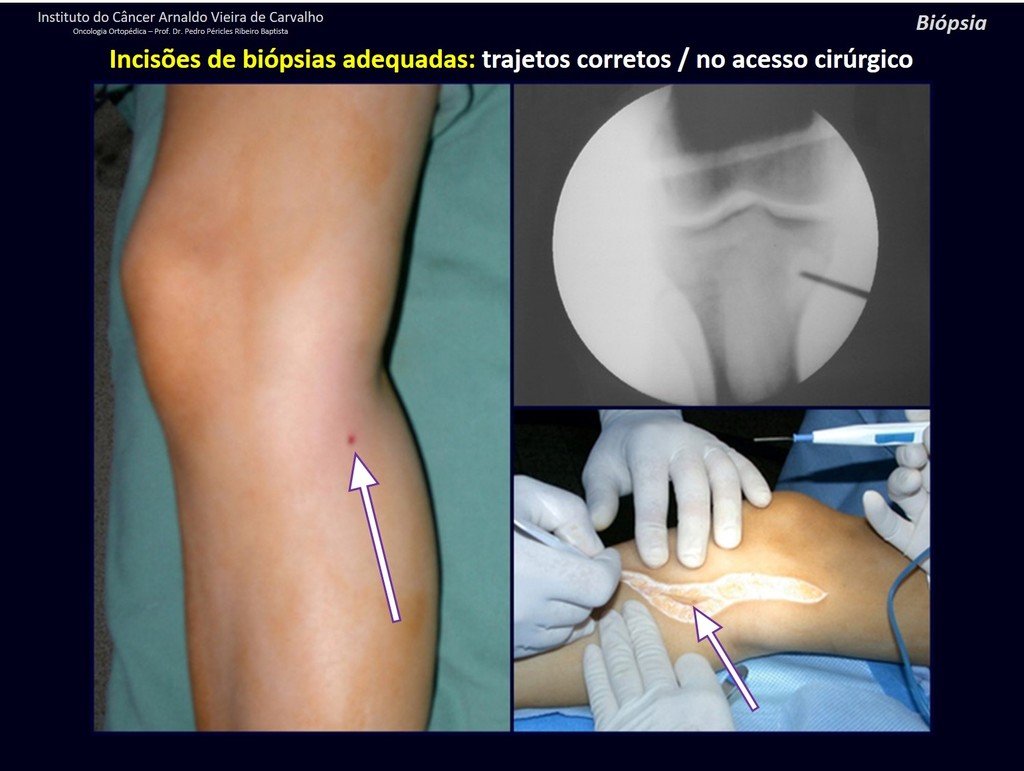
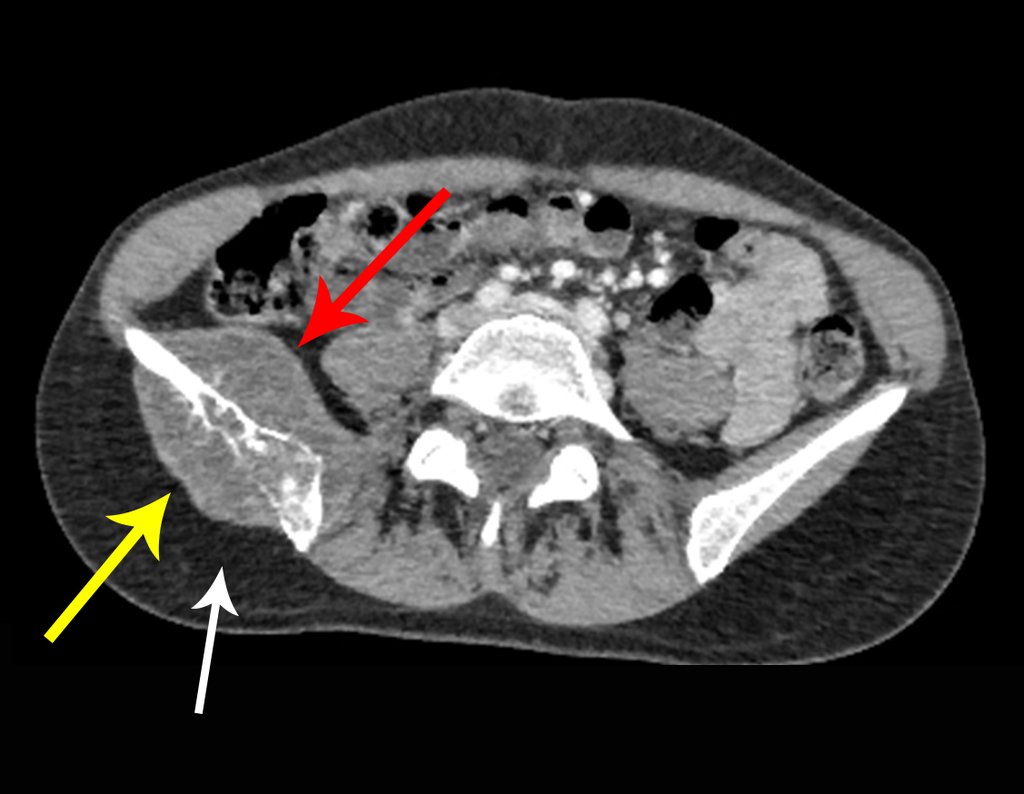
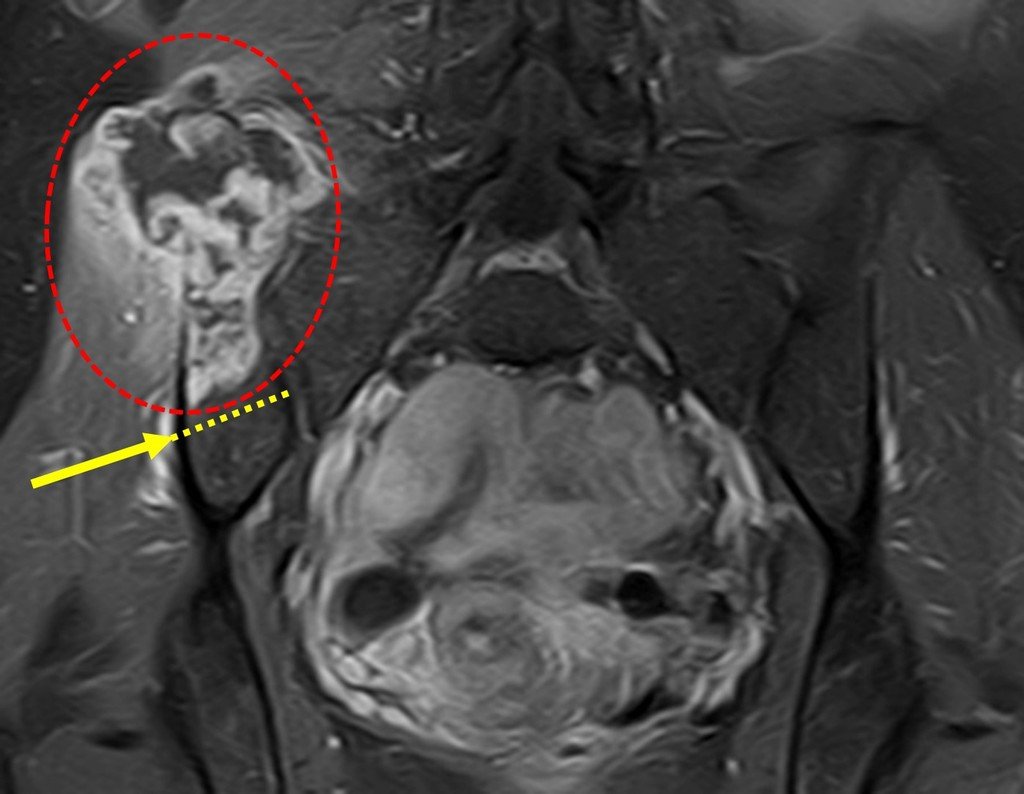
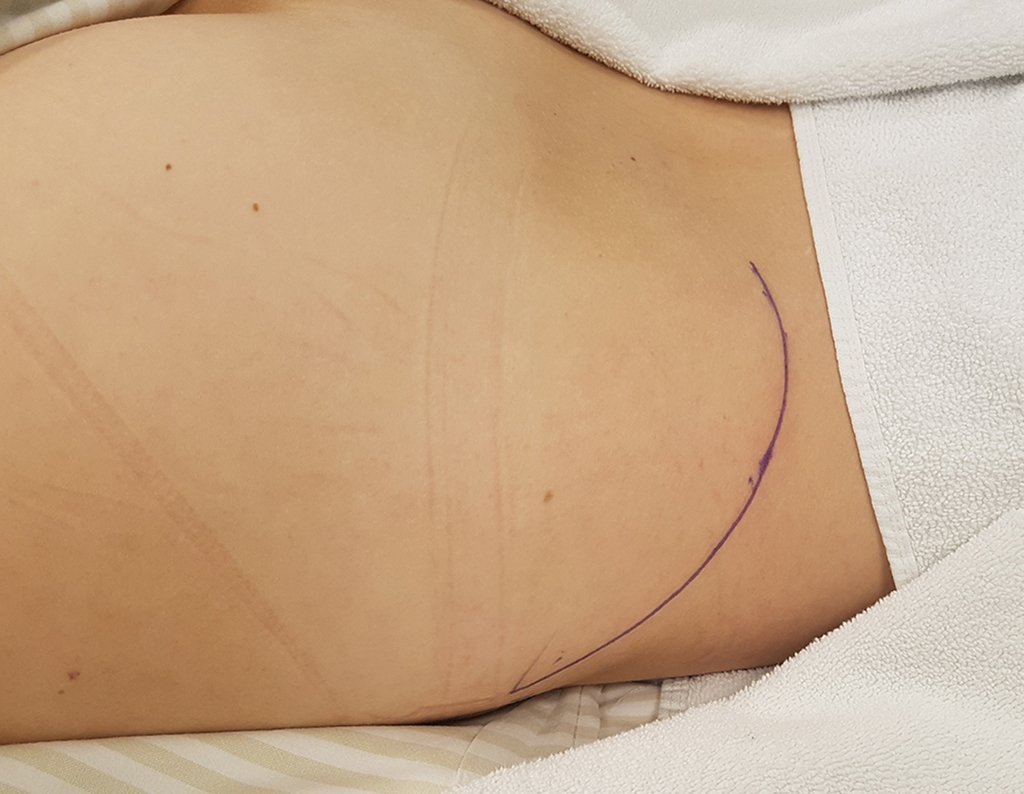
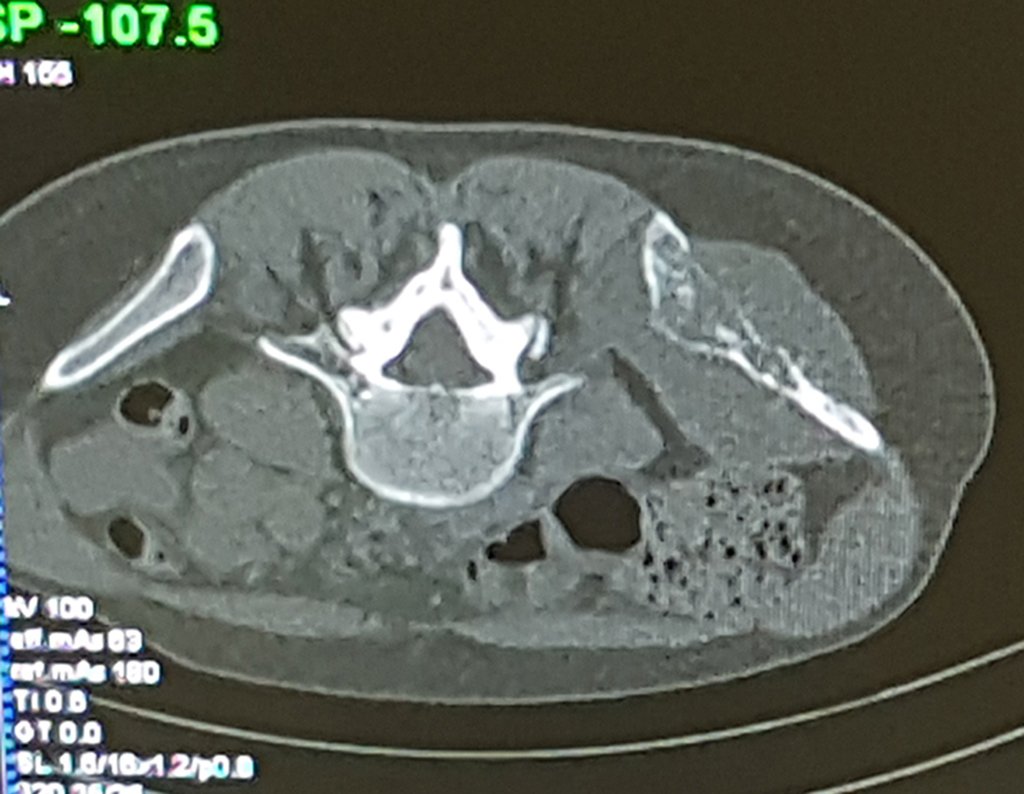
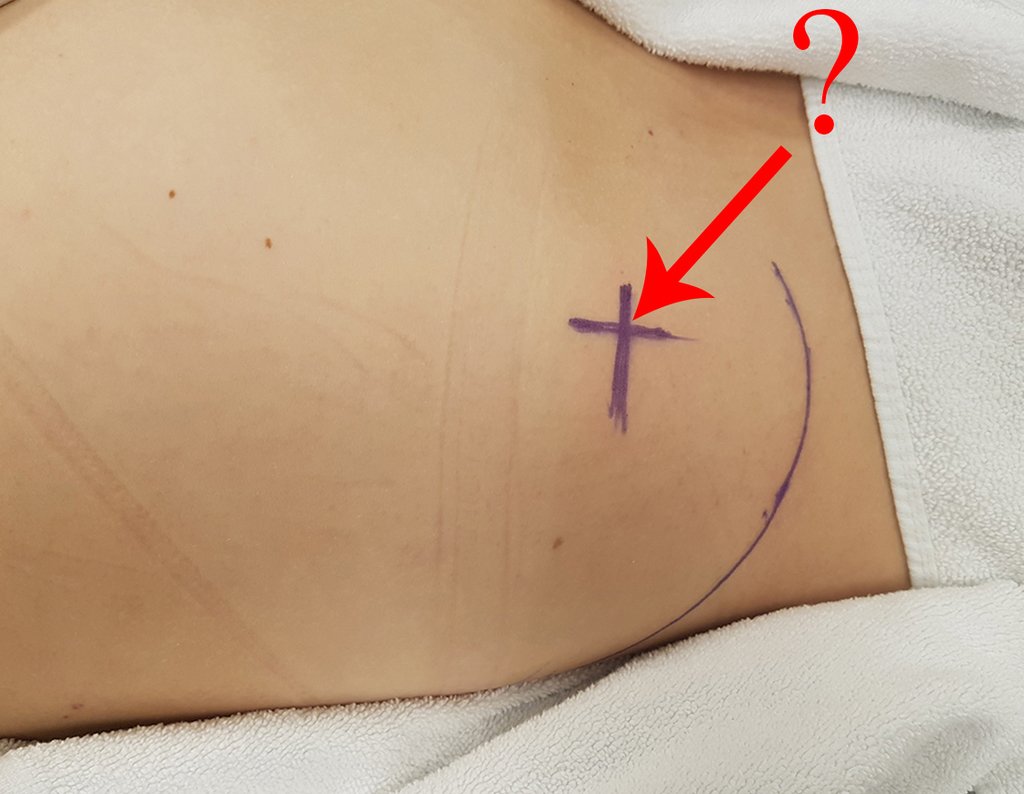
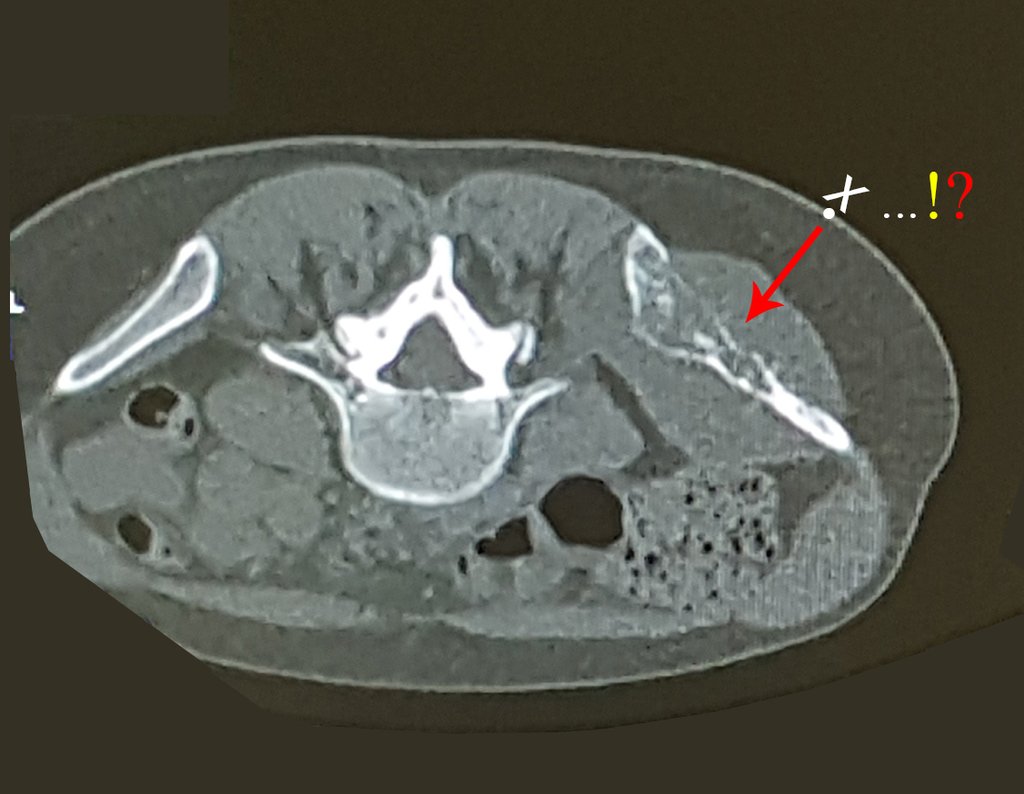
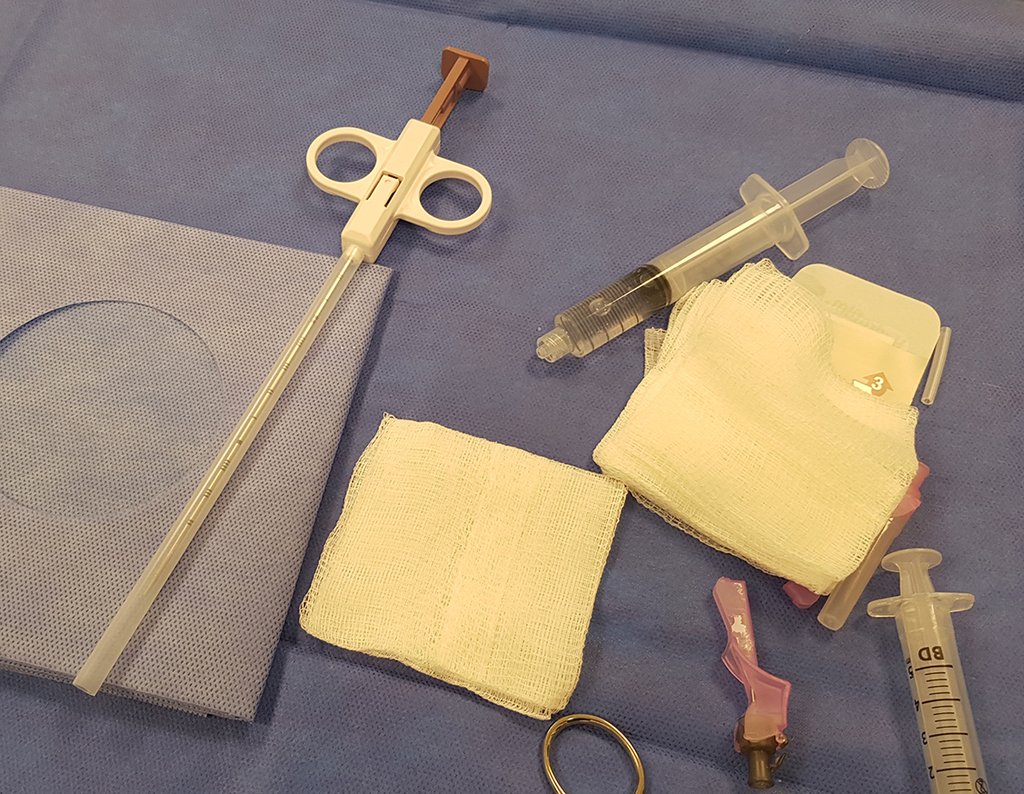
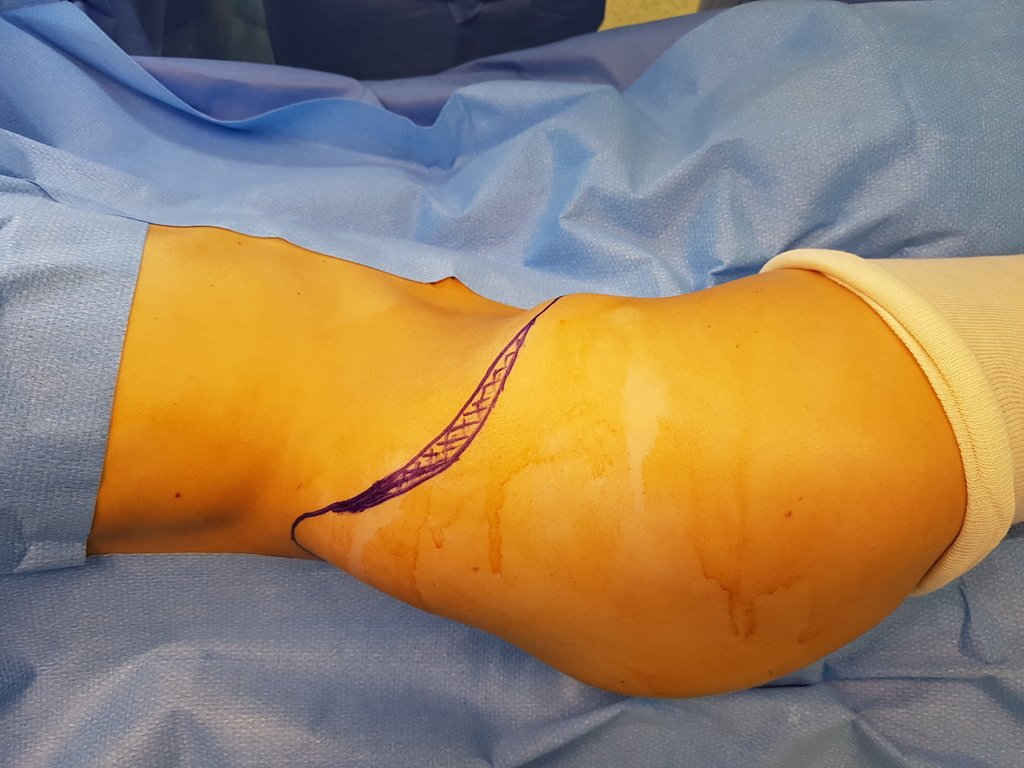
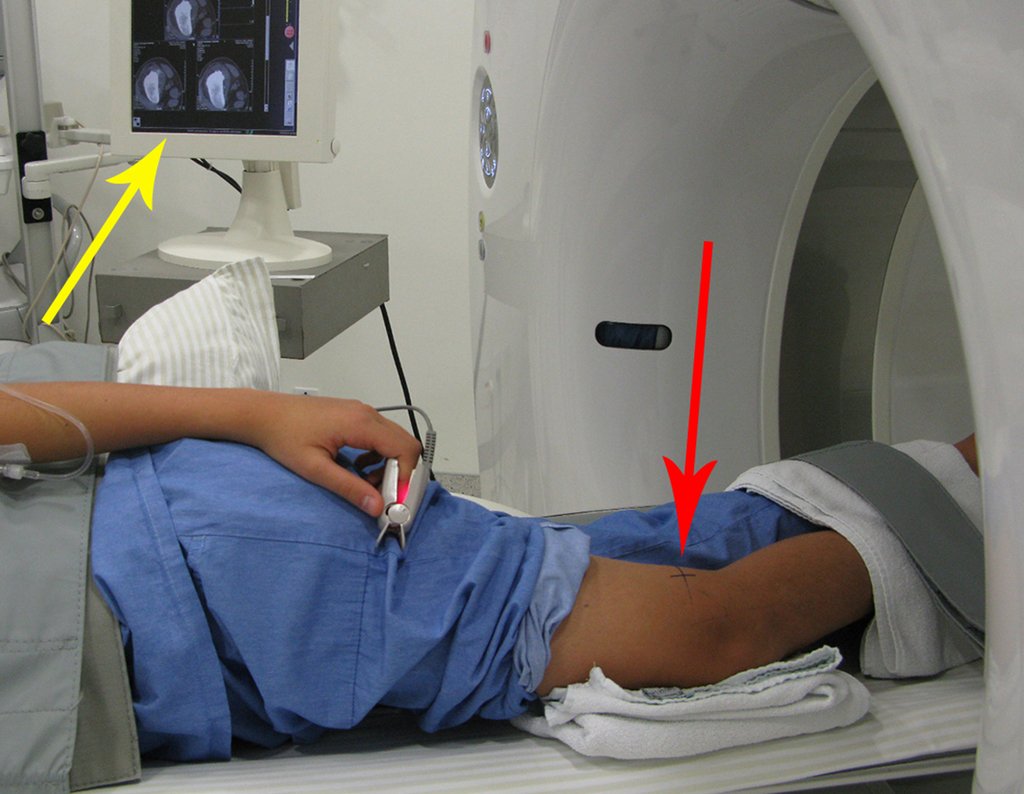
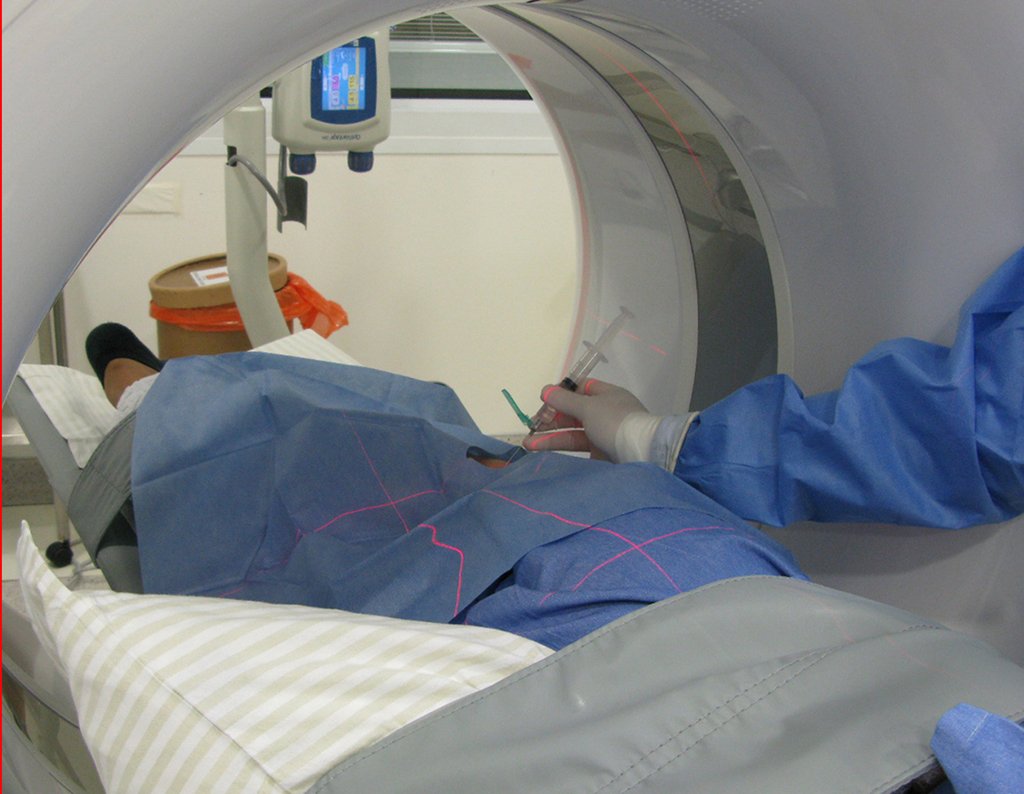
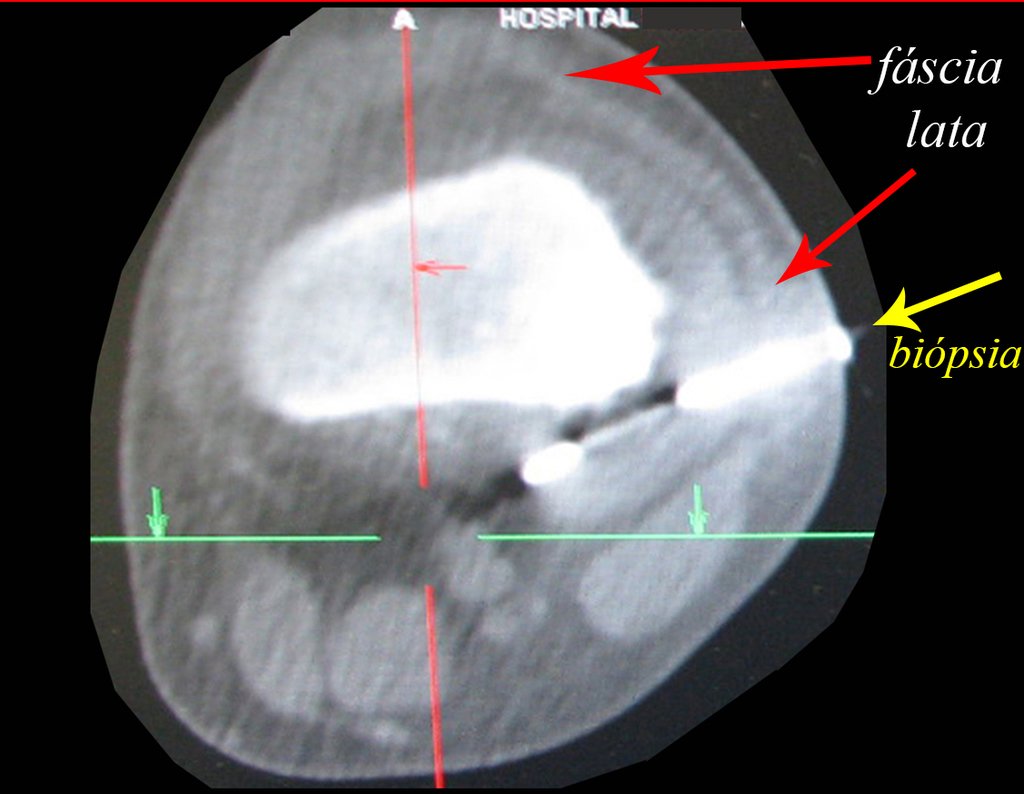
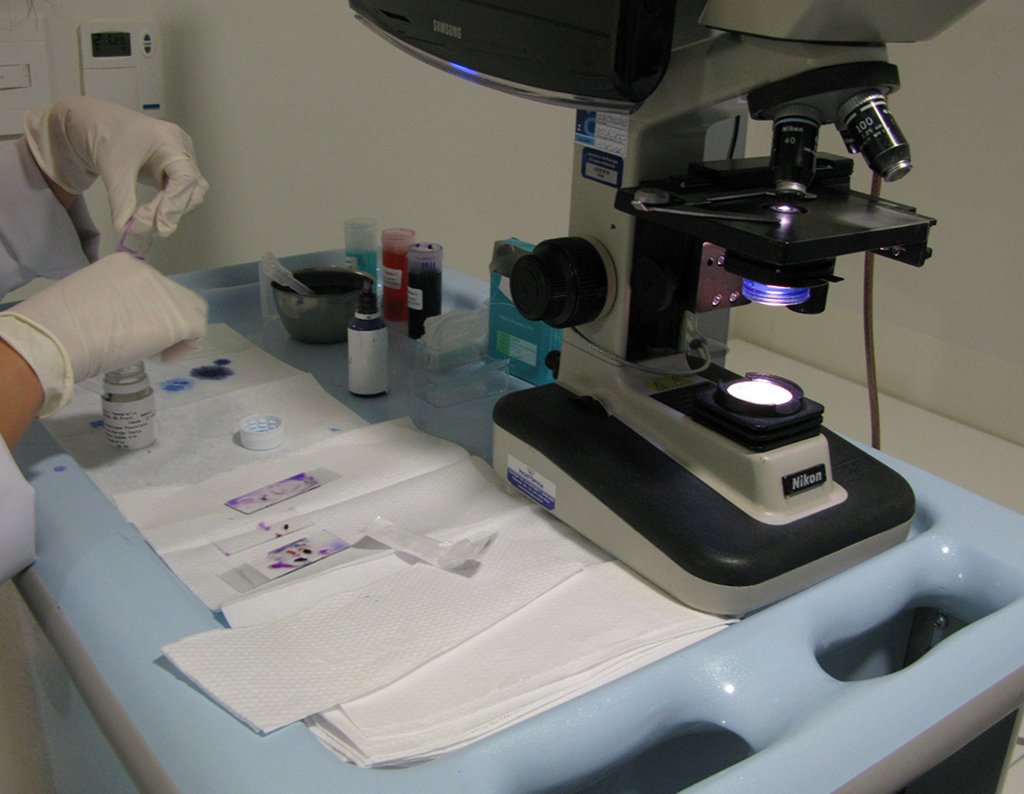
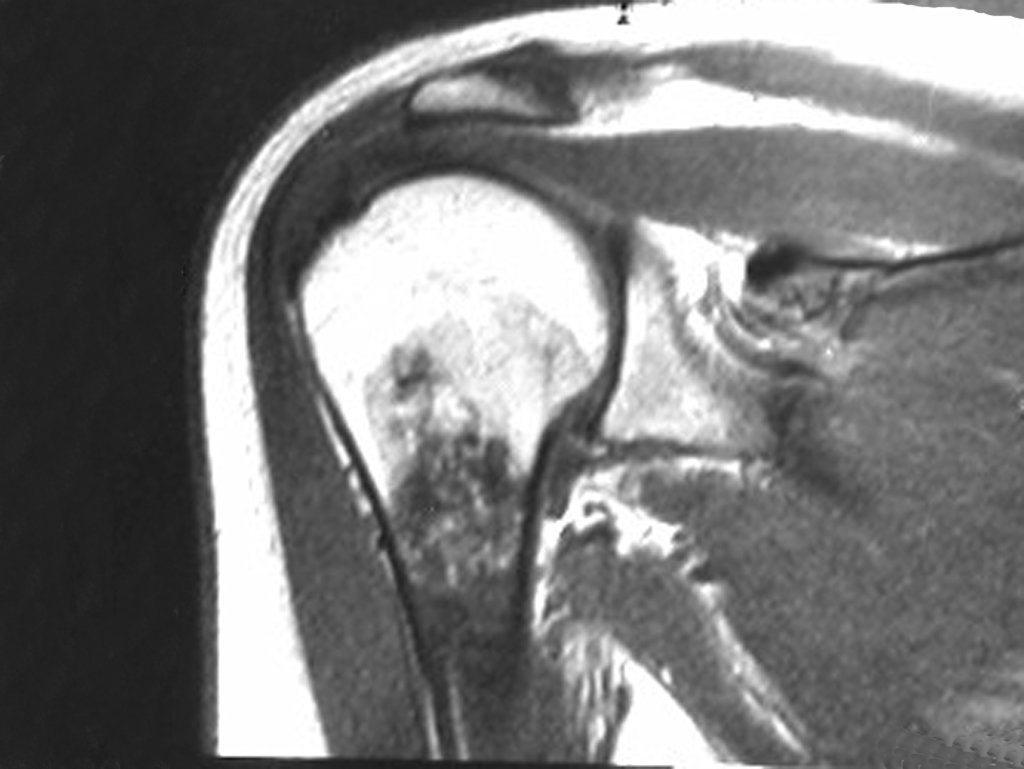
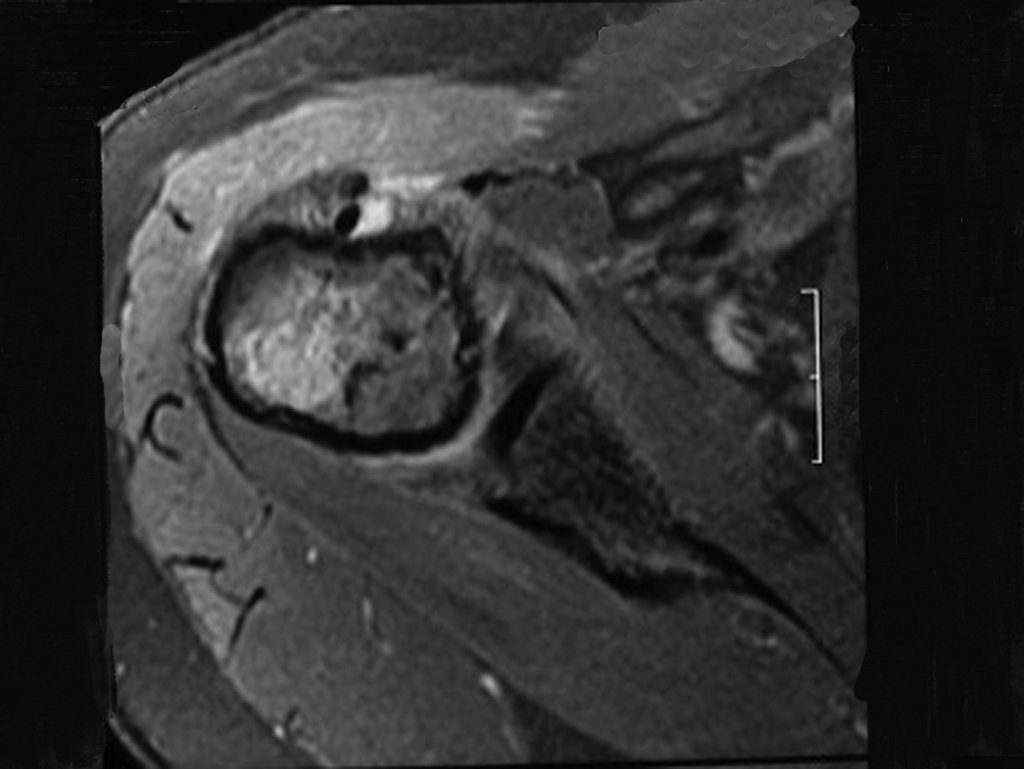
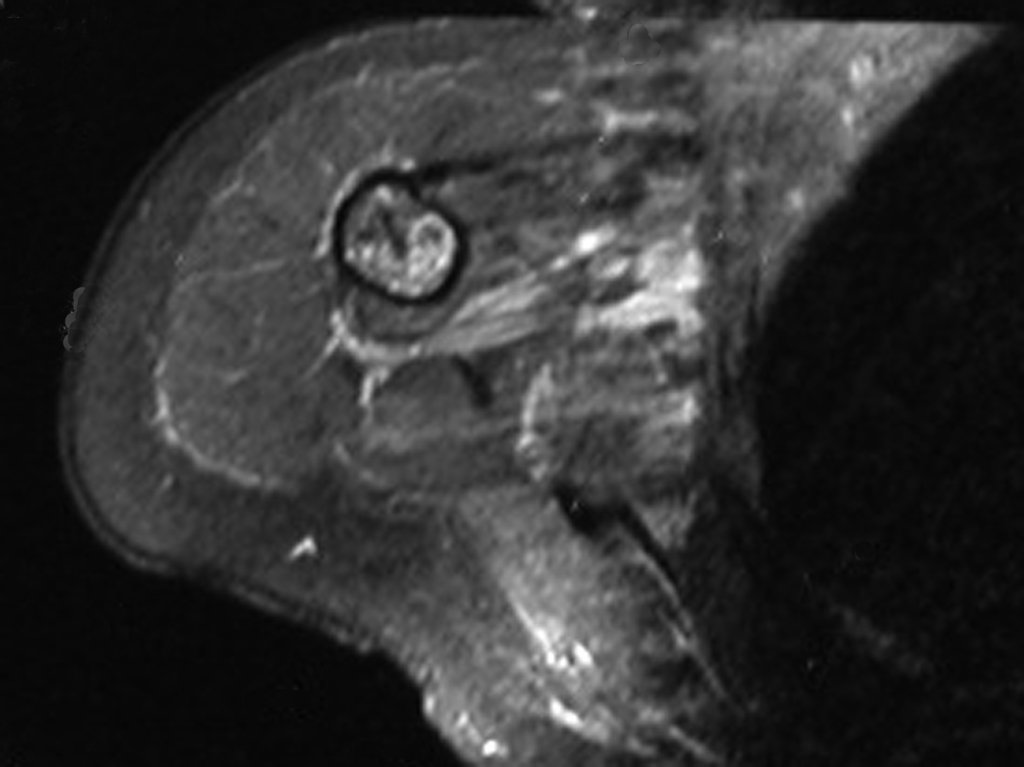
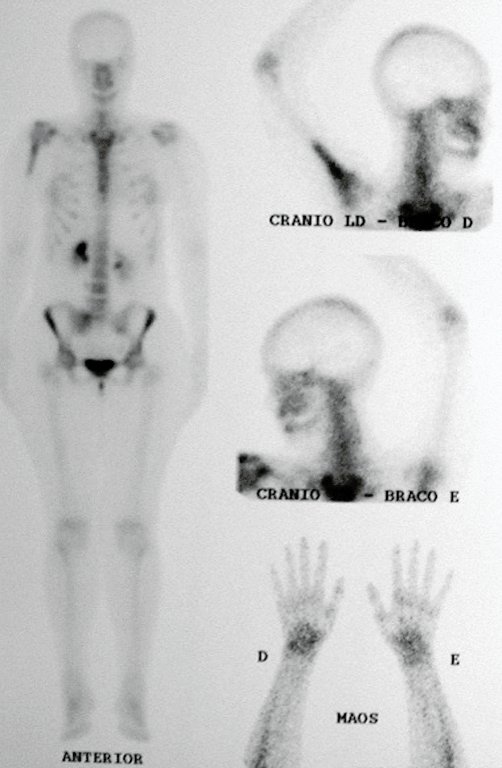
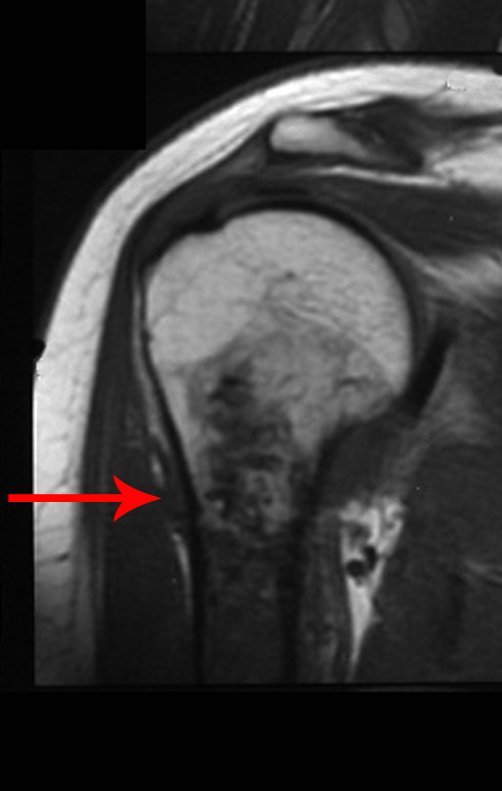
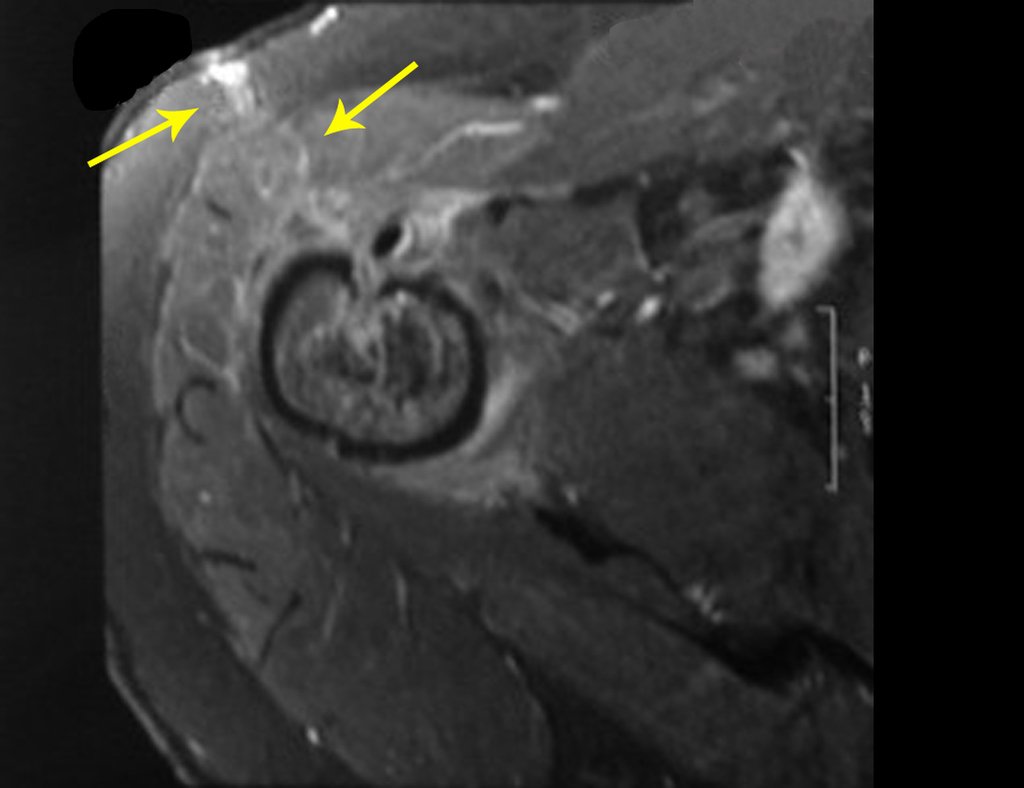
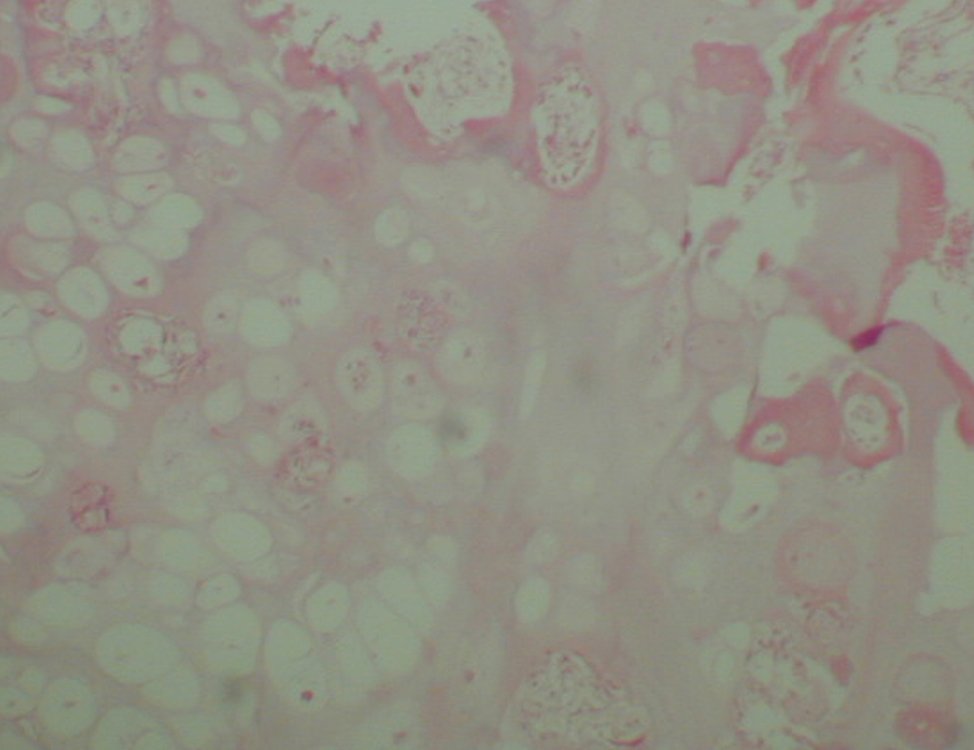
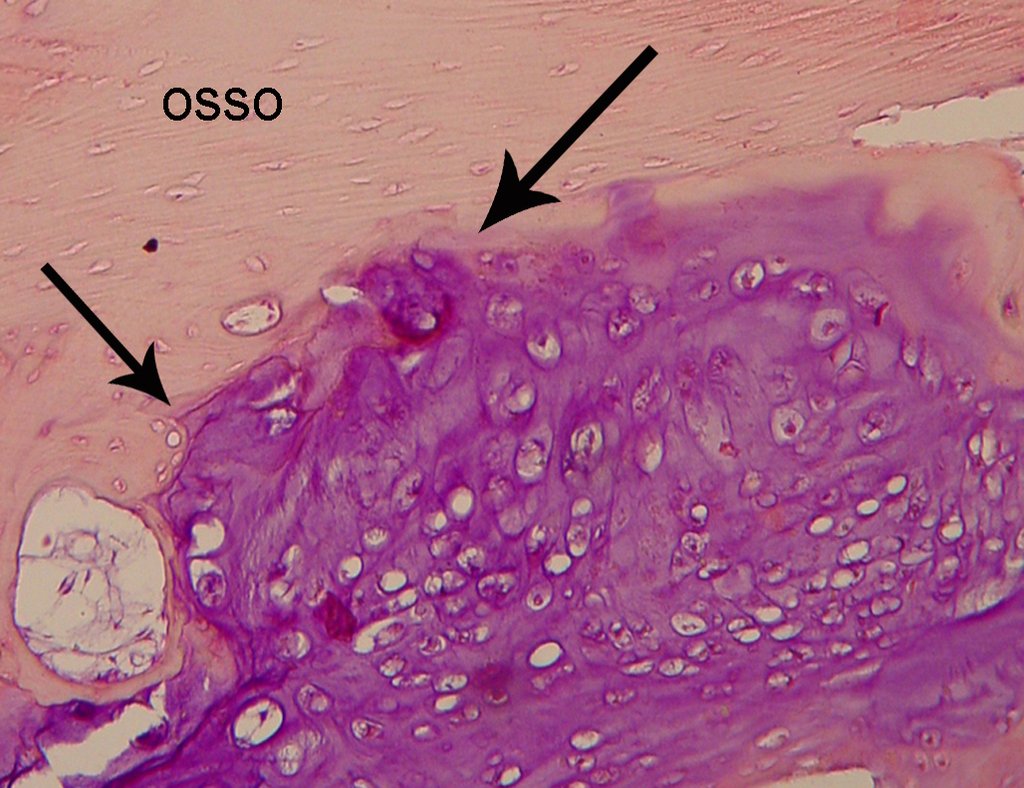
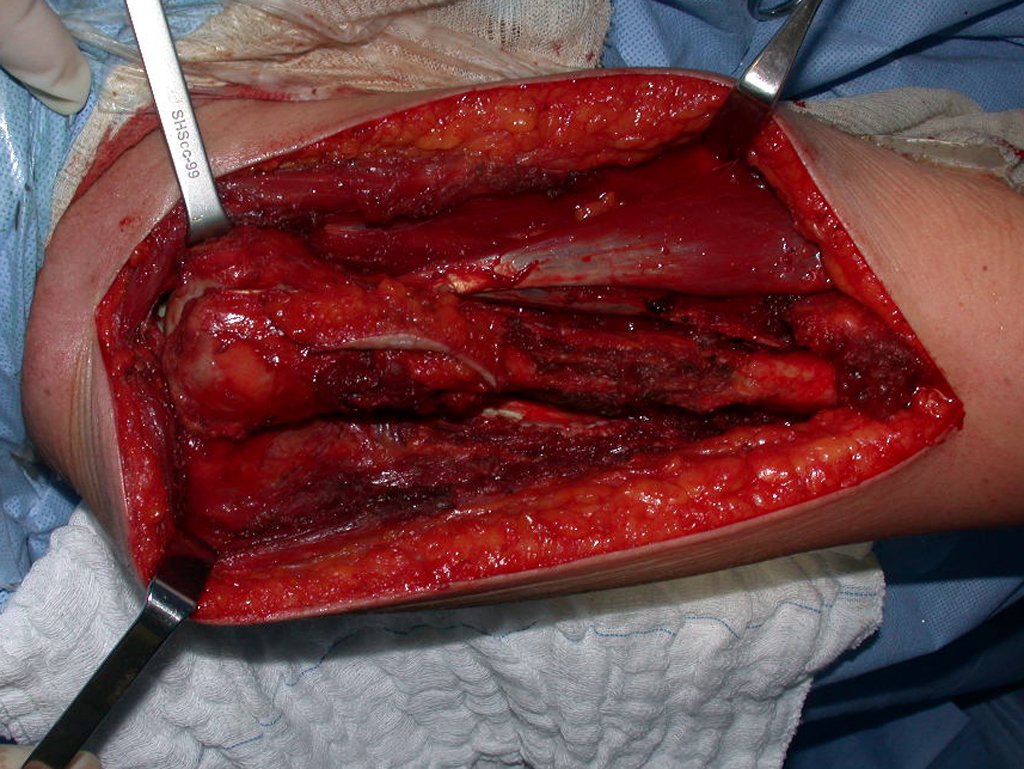
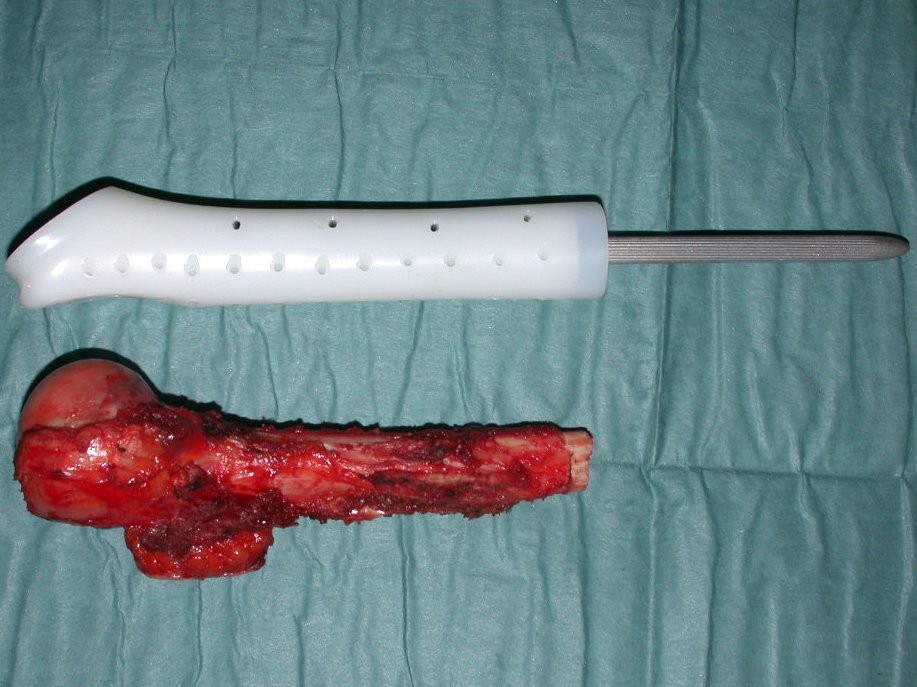
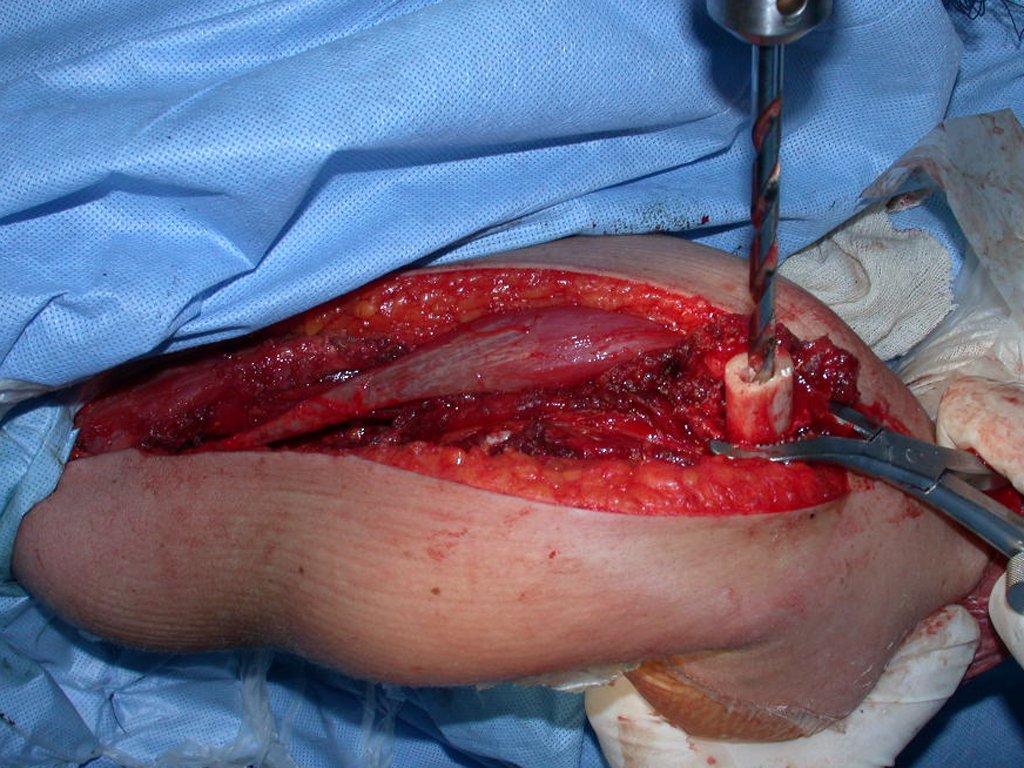
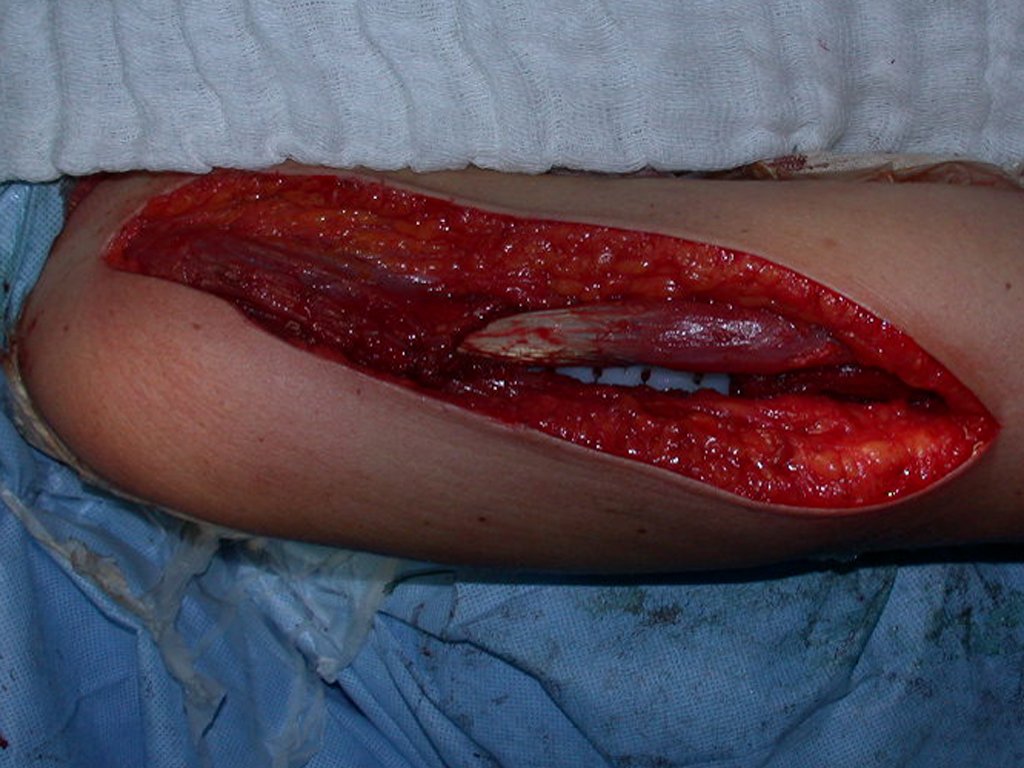
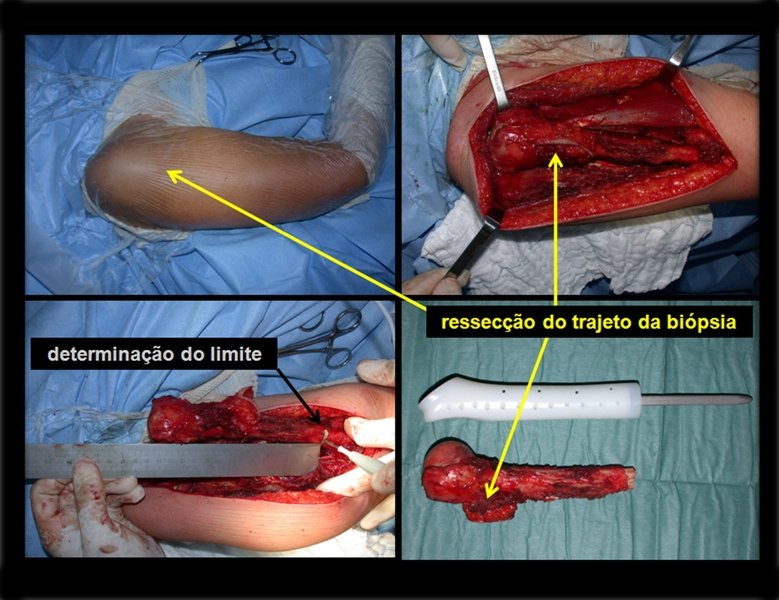
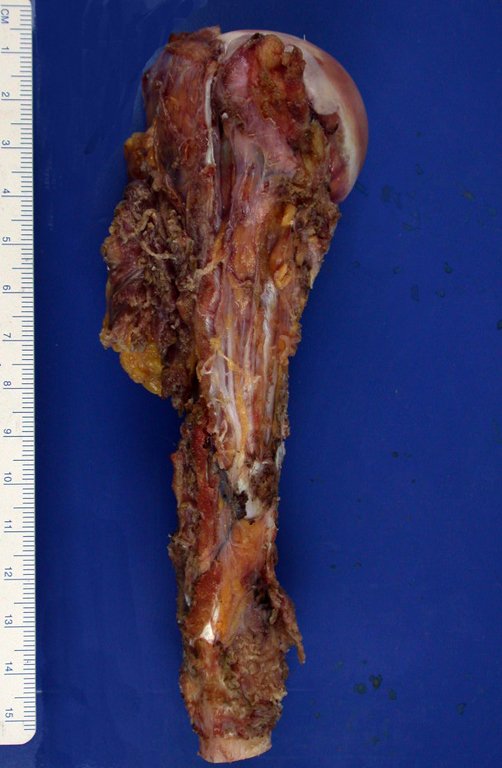
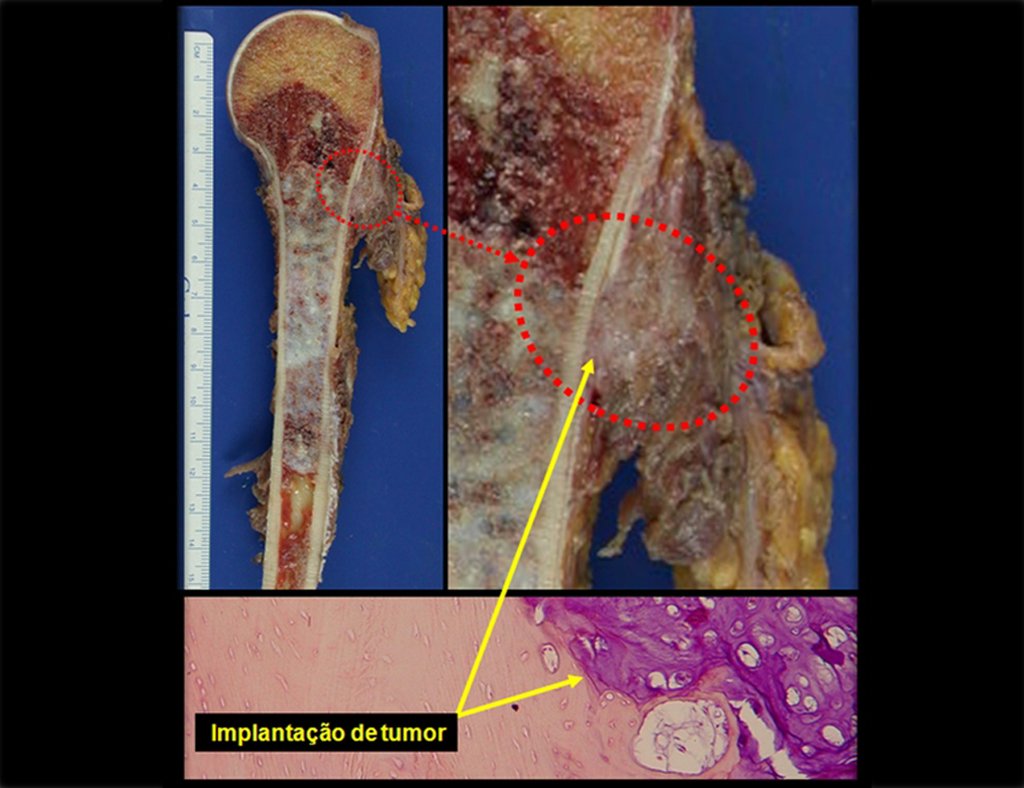
The patient has good function and plays very well, without any difficulty, their professional activities (Video 2).
REVIEW:
Chondrosarcoma is the primary malignant bone tumor more frequent after osteosarcoma 23,24). The central subtype is the most common and affects more than five times the periferic (3), there are also rare subtypes of clear and mesenchymal cells (2).
Normally arises in the bones of endochondral origin and mainly at the root of the limbs (shoulder, pelvis, rib and spine (1)) are rare in the membranous origin (24,11,15,14). It is slow growing and often the patient seeks treatment when the lesion presents major. This tumor can affect any age, with prevalence between 30 and 40 years (7, 11, 22), with reference in the literature since three years (15) to 73 years old (1).
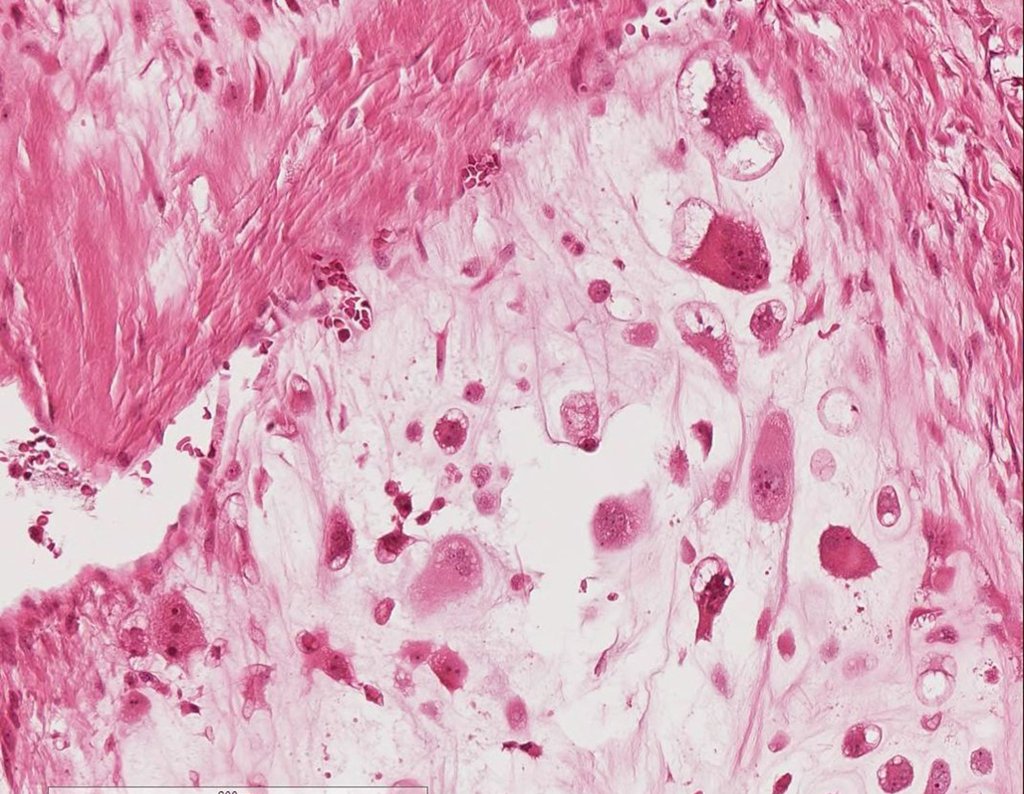
It is a malignant tumor of mesenchymal nature, producing interstitial substance and cells that assume aspect of hyaline cartilage, with varying degrees of immaturity and frequent calcification foci and can occur in different locations.
Can be classified according to location: A- Central , B- cortical fair (Paraosteal or Periosteal) (23,2,24,6,3), C-Peripheral (or exophytic, which occurs in the cartilaginous cap of an osteochondroma) (28) and D- Soft Tissue Chondrosarcoma (13); on the histology in: A- degree of anaplasia: are classified into grades I, II and III, dedifferentiated; B-, C- and D-:mesenchymal clear cell; as to the origin may still be: 1 primary and 2 secondary (which originates at the site of a preexisting benign cartilaginous lesion as in Oilier disease (Enchondromatosis) or Maffucci syndrome processing for Chondrosarcoma is common (20 to 30 %) (2.28), can also occur as Solitary Osteochondroma (in less than 1%, or multiple 10%) (2), and more rarely secondary to Paget’s disease.
Pain can be insidious symptoms for several years, progressing to slow growth, volume increase, mobility restriction getting skin sometimes reddish and warm (23). The first symptom is often a fracture in pathological bone (2.24).
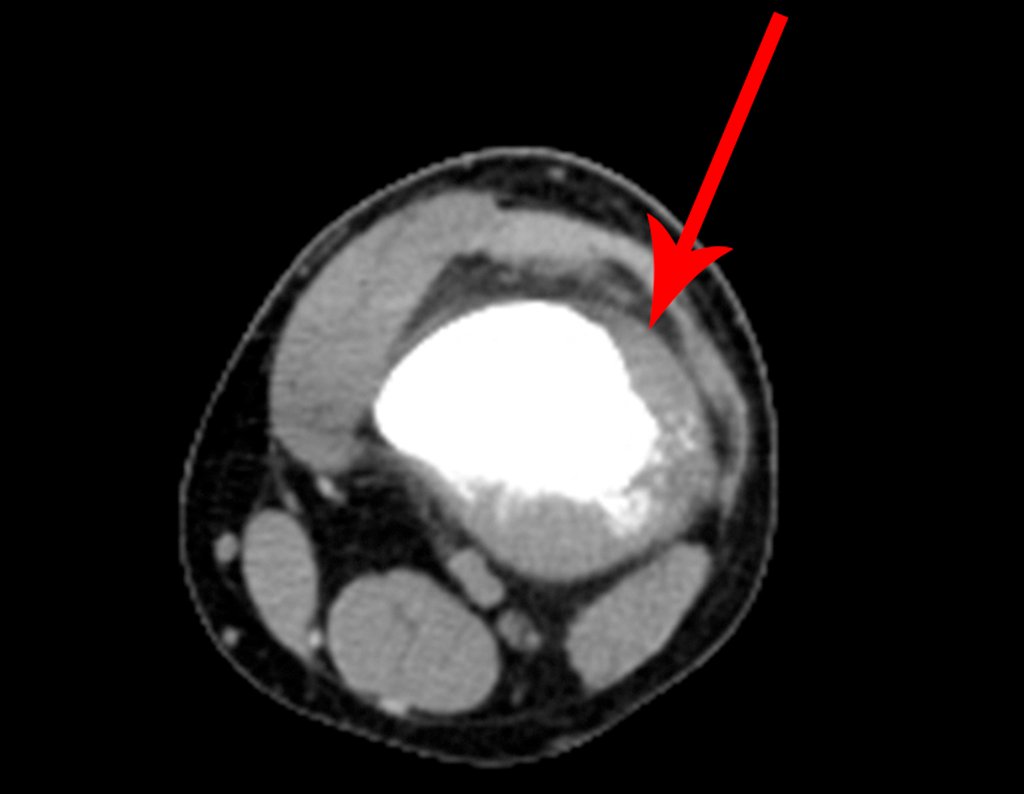
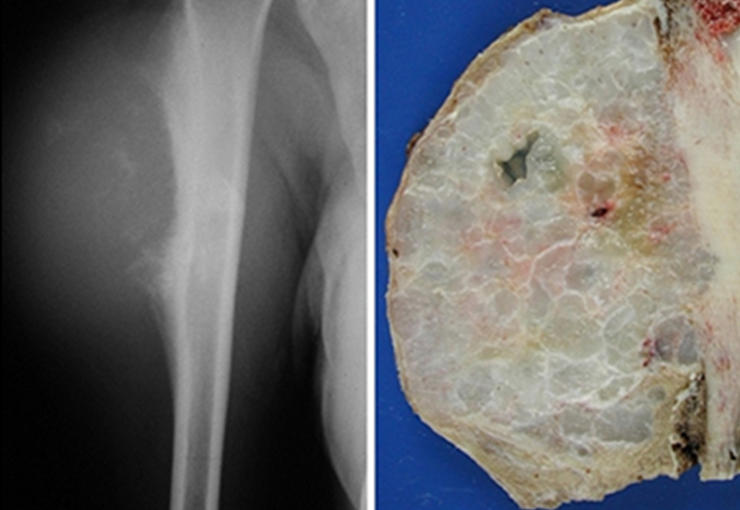
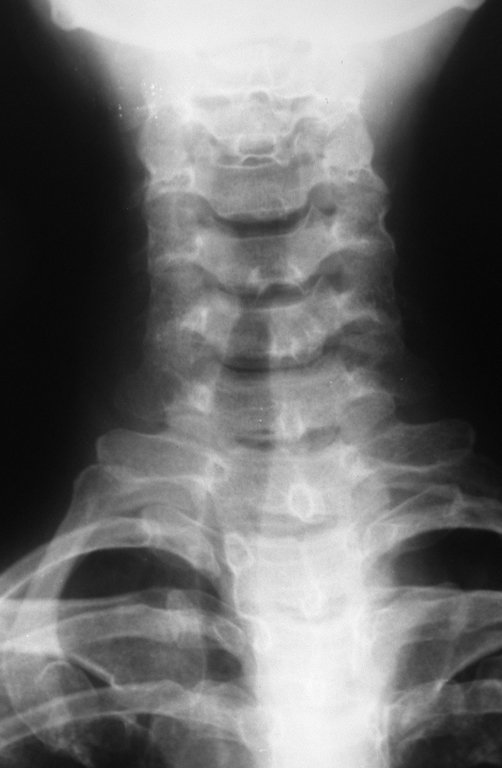
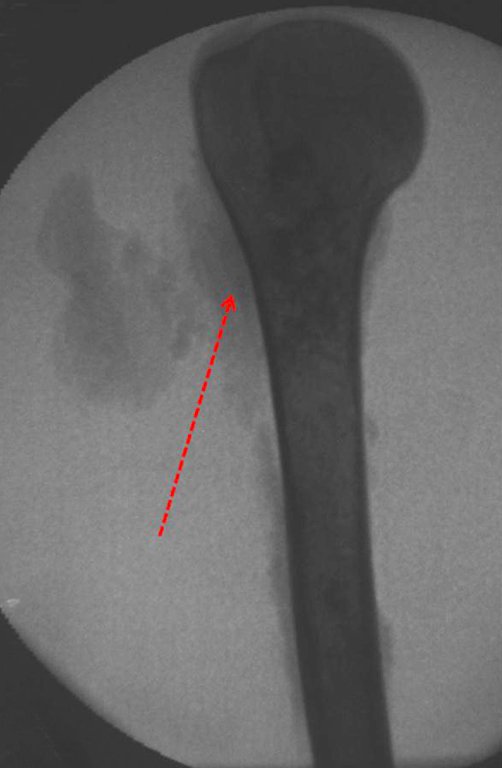
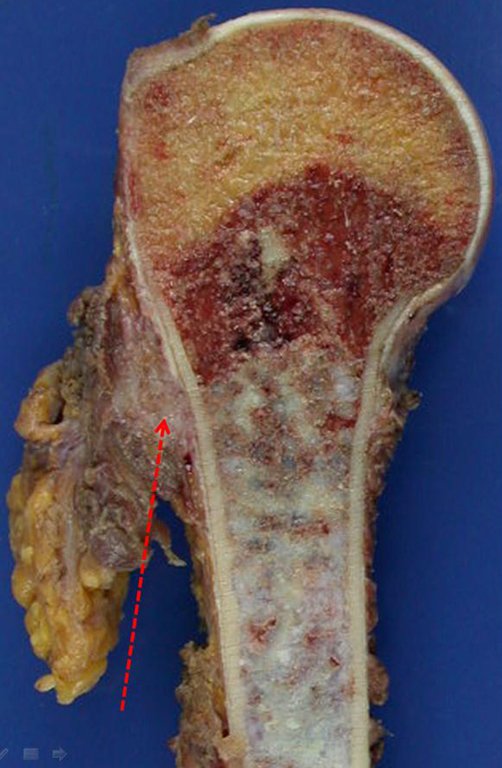
The radiograph shows transparent radio metaphyseal lesions replacing the bone marrow that extend into the epiphysis or diaphysis , eroding the internal cortical ( lesions in piecemeal ) , inflating or expanding the medullary portion of the bone , but remaining bounded by cortical that thick.
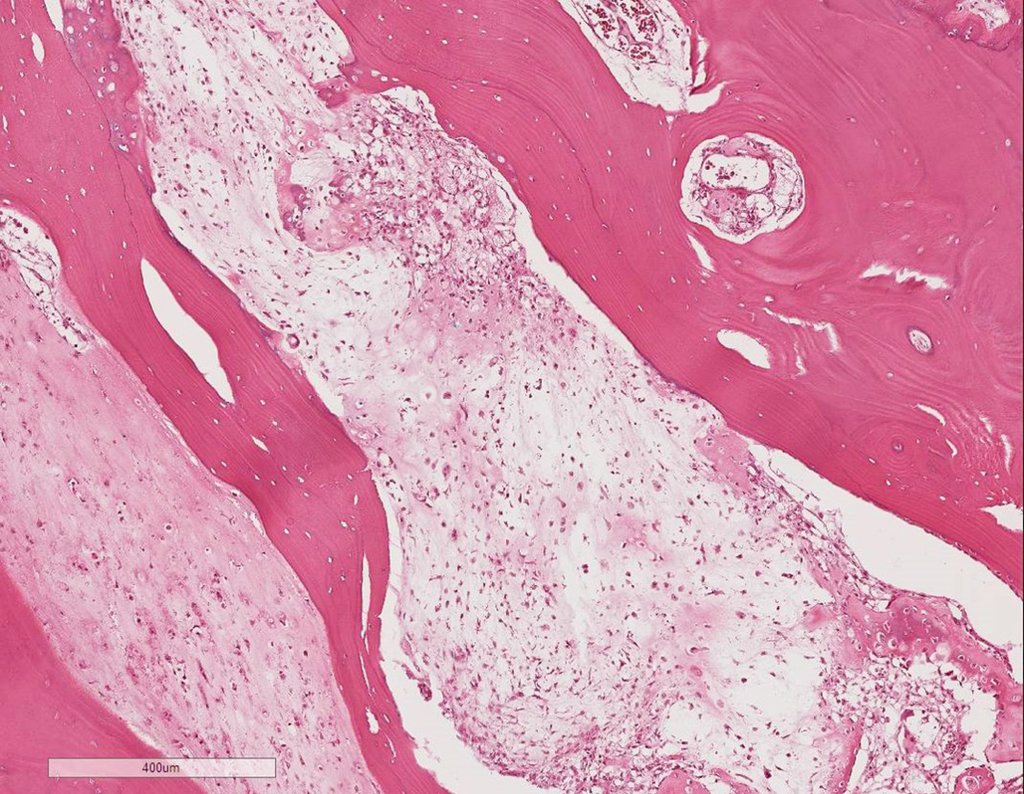
The appearance of calcifications ( dotted such as cotton balls ( 5 ) or rings ) is frequently (23, 2 , 24, 13 , 6, 28) . These are due to the degeneration of the cartilage that receives new vascularization and calcified . This process is accelerated in Chondrosarcoma and slow in benign cartilage lesions and low grade.
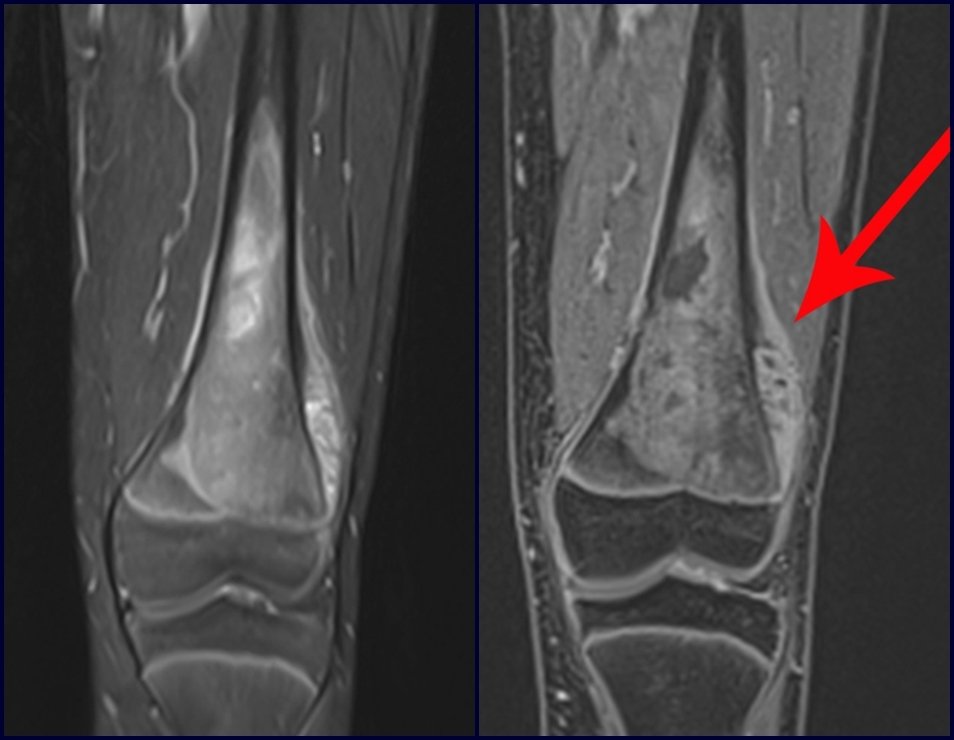
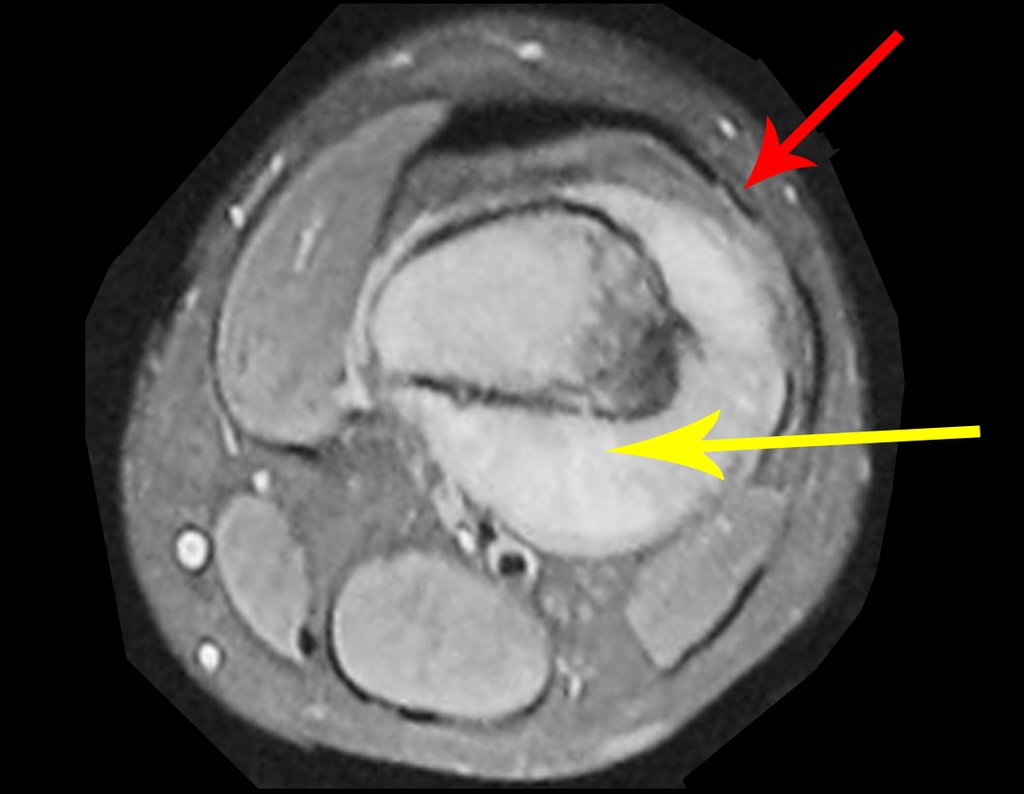
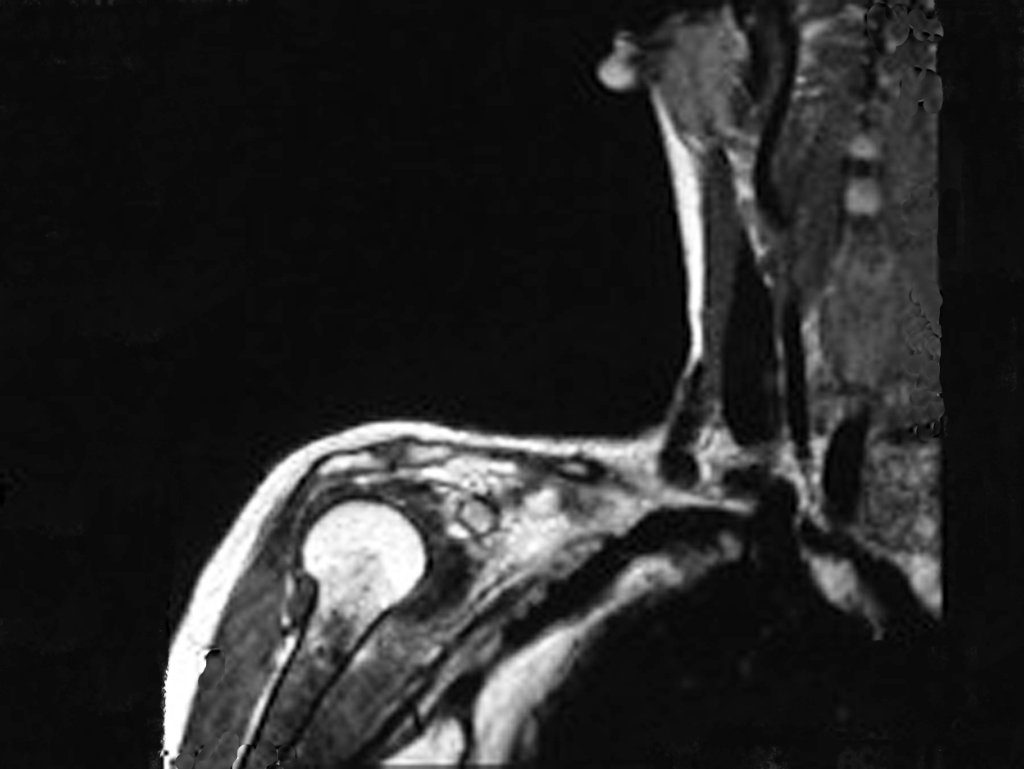
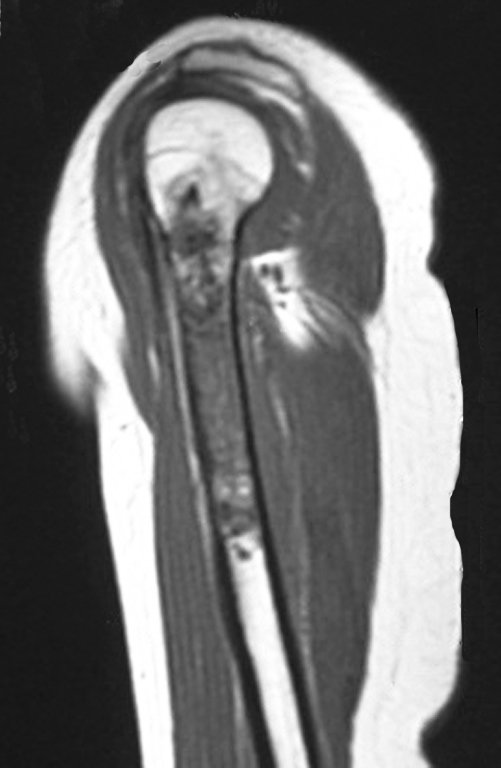
The bone mapping assists in lesion staging. MR and CT are important for the evaluation of the intramedullary extension and extra osseous lesion ( 2).
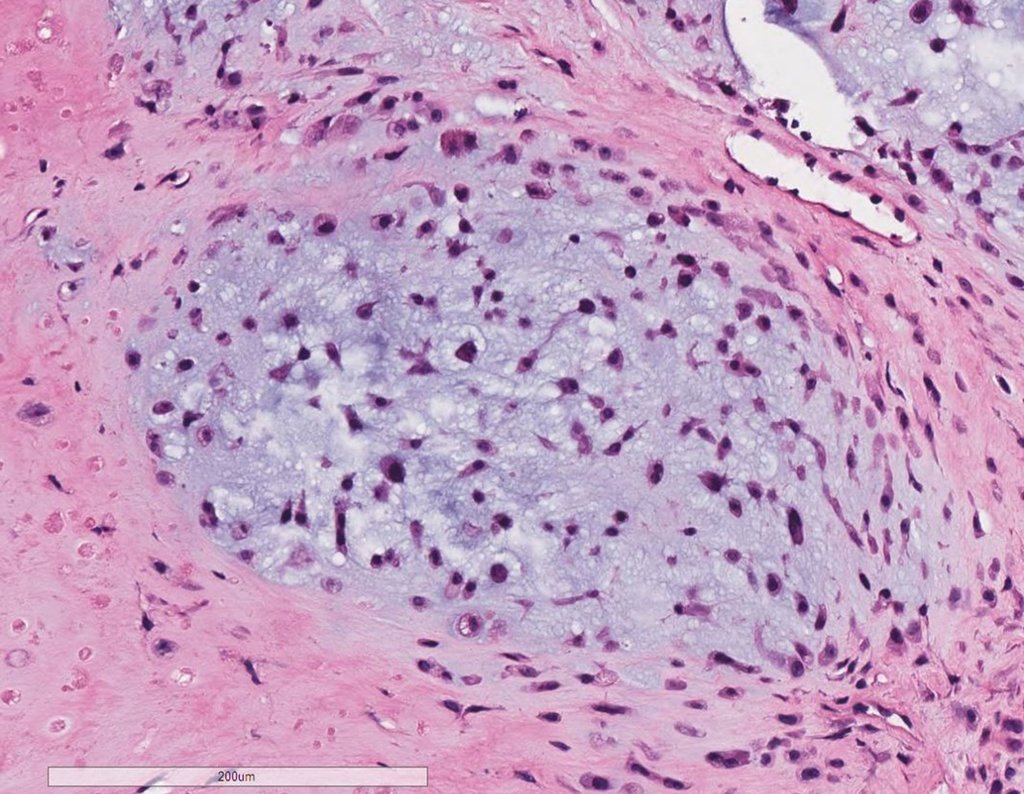
The diagnosis of well-differentiated Chondrosarcoma presents difficulties and histological data from clinical history, location, imaging findings should be valued for diagnostic conclusion and definition of proper behavior (23, 14, 12 ) . The irregularity of histological minutiae in the array and the number of cells within the chondroid matrix, the core of hyperchromasia changes , polymorphism and atypical mitotic when located in members of roots must be considered grade I Chondrosarcomas , although these same histological aspects can be found in benign Chondromas of hand and feet. In microscopic descriptions are similar to Central Chondrosarcomas ( 23).
For the diagnosis we must also differentiate the pathological , clinical and radiological similarities with other injuries.
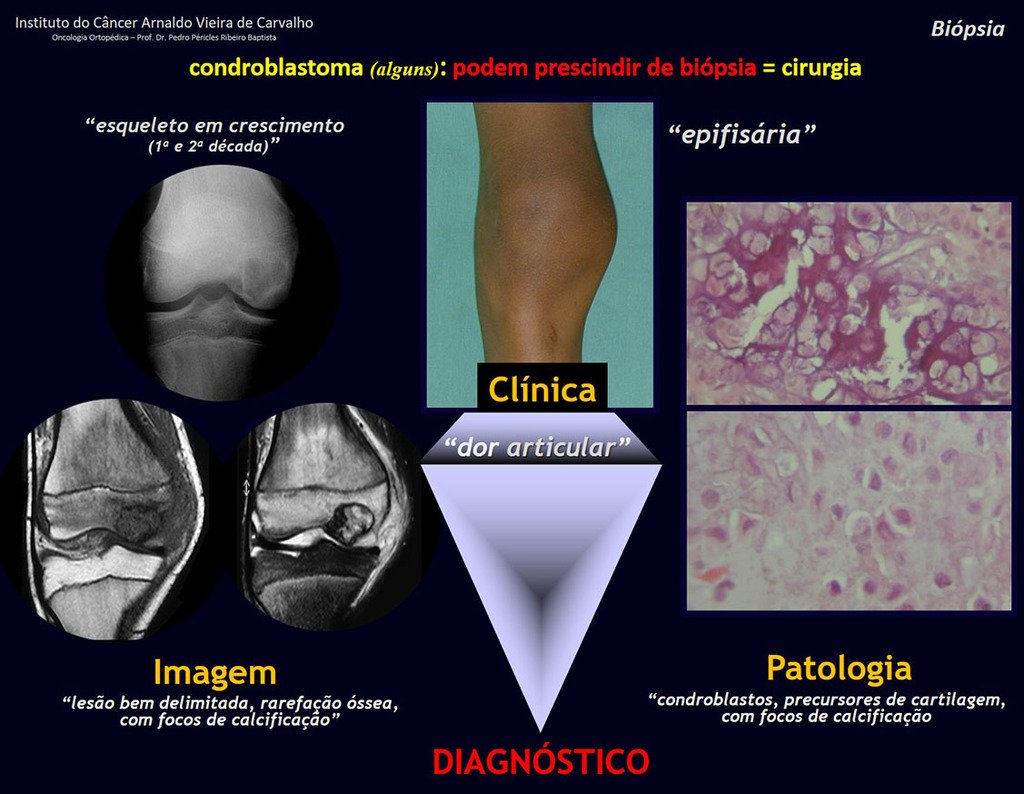
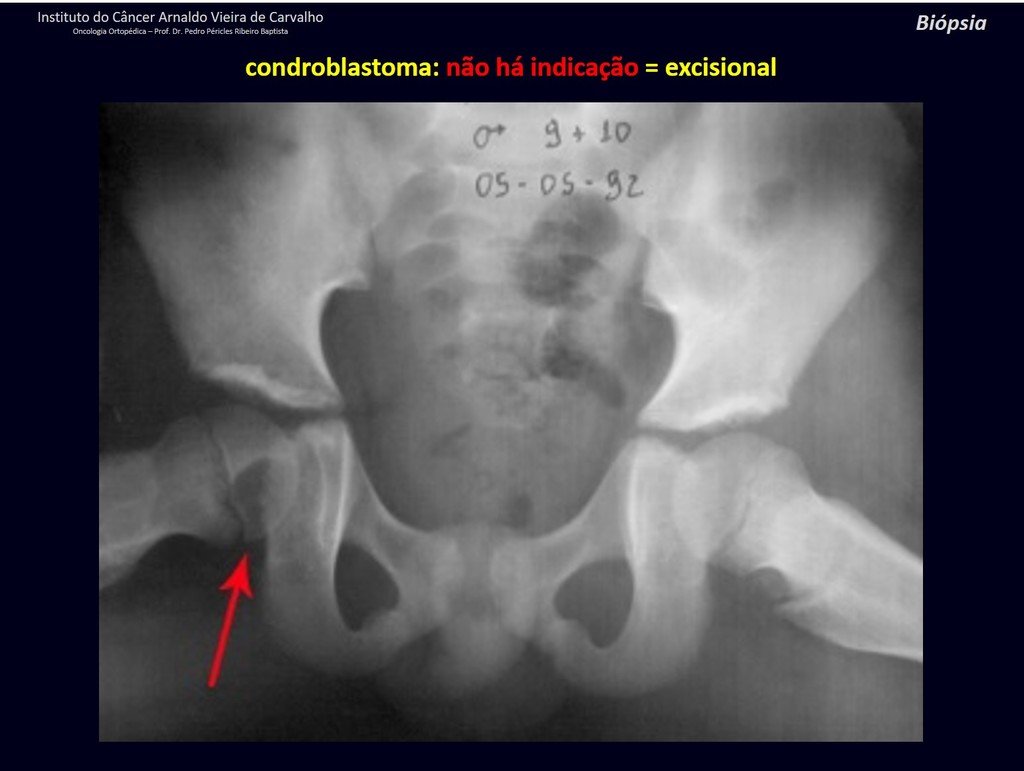
The diferential diagnosed with aneurysmal bone cyst is on the multiloculated character; with Chondroma , the Osteochondroma , Chondroblastoma , the Paraosteal and Periosteal Osteosarcoma (with cortical just Chondrosarcoma ) ( 16); Myositis Ossificans ; Fibroma Chondromyxoid ; T.G.C. and Non-Hodgkin Lymphoma ( 23 , 6, 28) . The Clear Cell Chondrosarcoma has intra lesional formation of reactive bone may cause confusion with Osteosarcoma . The Mesenchymal Chondrosarcoma is formed by small round cells that resemble Hemangiopericytoma and Ewing’s sarcoma ( 14). The central Chondroma of long bones, Chondrosarcoma and Bone Infarction are often difficult to diagnose , requiring periodically clinical and radiographic evaluation to monitor the evolution of injury and definition of conduct.
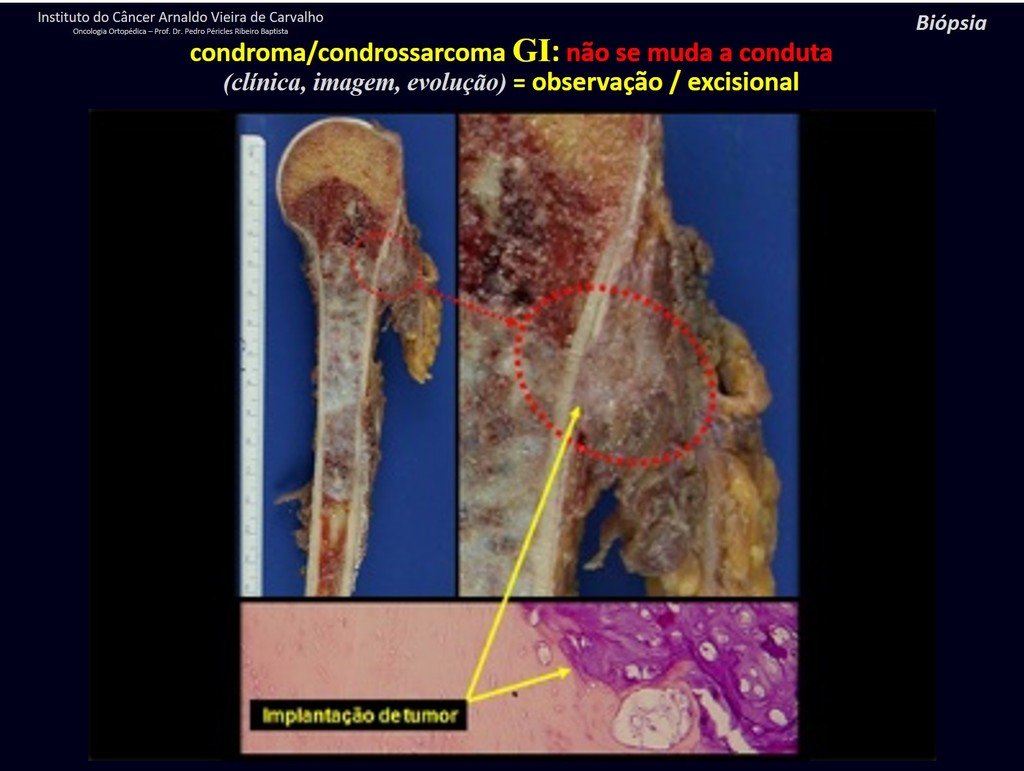
A biopsy can often not definitive for diagnosis ( 23, 28 , 12).
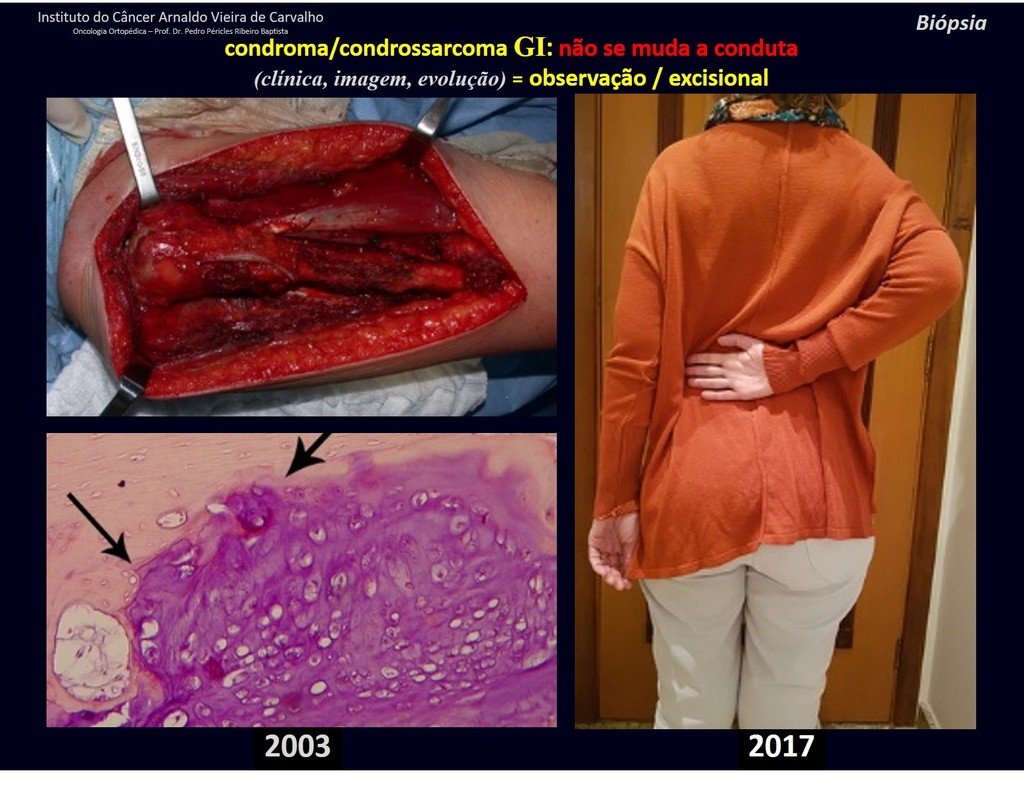
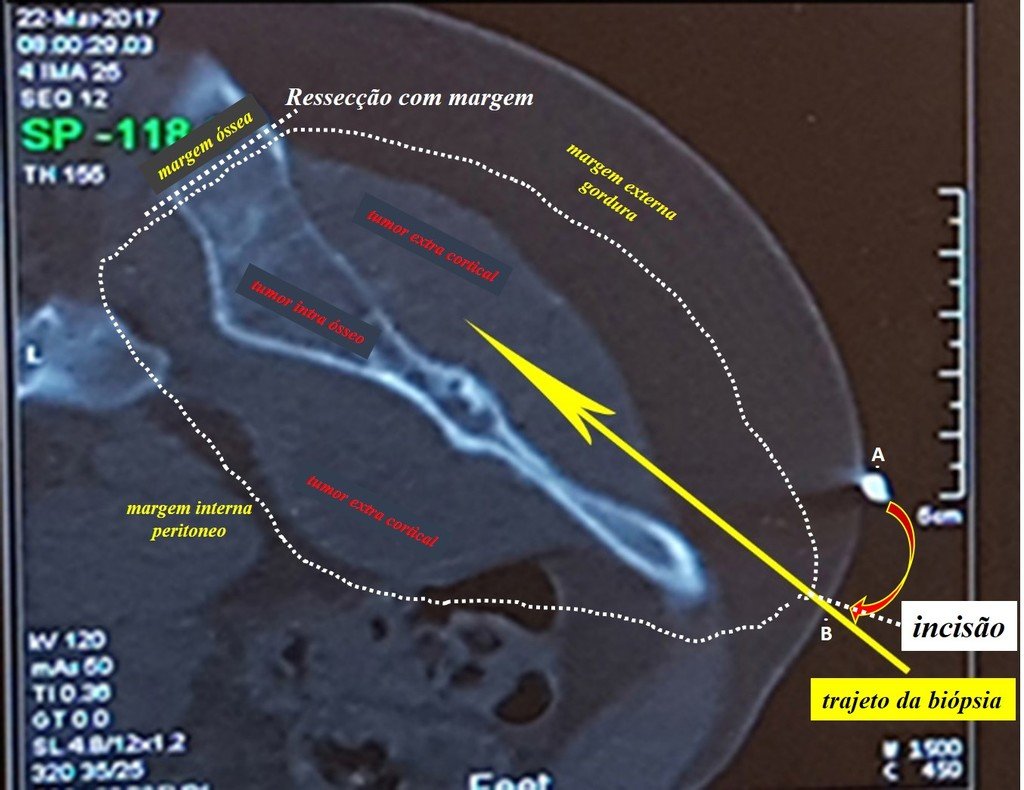
The treatment of Chondrosarcoma is exclusively surgery (25 ) , should be chosen a wide resection , including the path of the biopsy (21, 13). Radiation therapy is ineffective ( 6) in controlling this cancer. For cases of grade III can be argued chemotherapy indication to the protocol used for large cell high-grade sarcomas . Mesenchymal Chondrosarcoma in which present predominance of small cell undifferentiated , discussed the chemotherapy lies with the treatment protocol of Ewing ‘s sarcoma. In both cases the response to chemotherapy is usually poor ( 6). The treatment of this cancer should be individualized for each clinical subtype.
Complications occur hematogenous metastases to the lungs ( 28) , may also present lymphatic dissemination and local recurrence. Many chondrosarcomas feature local invasion trend (14 ), reaching enormous sizes becoming inoperable and causing death by this local complications propagation.
The local recurrence increases the incidence of lung metastases ( 21).
EXERCISES:
1- What are the radiographic features of Central Chondrosarcoma ?
a) Intra and extra medullary ossification.
b) Diaphyseal lesion with bone thinning and triangle of Codman with coarse lamellar reaction.
c) Areas of bone thinning , internal cortical erosion and calcification foci.
d) Bone condensing areas with periosteal reaction onion skin.
Answer: c) the cartilaginous tissue is more radiopaque than bone and thus presents itself as causing bone thinning and enlargement of medullary lesions in the bone marrow cousing cortical erosion . This growing cartilaginous tissue gets vascular sprouts and cartilage comes into regression due to calcification.
2- What are the characteristics of the MR of Chondrosarcoma ?
a) hyper- signal in T1, T2 and low signal with contrast enhancement.
b) hypo- signal in T1, hypo- signal in T2 and capture the contrast.
c)hypo- signal in T1, hyper- signal in T2 and without contrast uptake.
d)low signal on T1, high signal on T2 and capture the contrast.
Answer: d ) the cartilage tissue has low to intermediate signal on T1 . Intermediate down the cartilage and the calcified foci . Displays contrast uptake by increasing the local metabolism due to cancer.
3- What are the main differential diagnosis of Central Chondrosarcoma ?
a) Bone infarction and chondroma.
b) Osteochondroma and Ewing’s sarcoma
c) Osteomyelitis and T.G.C.
d) Osteosarcoma and condroblastoma.
Answer: a ) Bone Infarction causes damage to the bone marrow , but does not cause erosion of the inner cortex and has no evolutionary character of pain. It is usually a diagnosis finding on occasional ray.The same applies to the Chondroma that does not evolve and is only cartilaginous remains of development.
4- What is the treatment for the central chondrosarcoma ?
a) Intralesional curettage and autologous bone graft.
b) Wide resection and replacement with nonconventional endoprosthesis.
c)Intralesional curettage , location adjuvant with liquid nitrogen and homologous bone graft.
d)Intralesional curettage , local adjuvant electrotherm and bone cement.
Answer : b ) The wide resection provides oncological treatment and reconstruction with nonconventonal endoprosthesis provides the best restoration of function.
5- Histologically it is difficult differential diagnosis between:
a)Osteosarcoma and Eosinophilic granuloma.
b)Grade I Chondrosarcoma and Chondroma.
c)T.G.C and Ewing’s sarcoma.
d) Osteoblastomas and Enchondroma.
Answer: b ) The central long bones Chondroma and chondrosarcoma grade I are often difficult to histological diagnosis , it requires the radiographic evaluation for the definition and conduct.
BIBLIOGRAPHY
ACKERMAN, L.V.; SPJUT, H.J. Tumors of bone and cartilage. Atlas of tumor pathology. Washington, Air Force Inst. Pathology, 1962, fasc, 4.
CANALE, S.T. Cirurgia ortopédica de Campbell. Barueri: Manole; 2006
DAHLIN, D.C. Tumores óseos . Barcelona: Ediciones Toray S/A; 1982
DORFMAN, H.D.; CZERNIAK, B. Bone tumors. St Louis, C.V. Mosby Co., 1997, cap. 7, p.410.
EDEIKEN, J.; HODES, P.J. Diagnóstico radiológico de las enfermedades de los huesos. Buenos Aires, Panamericana, 1977, cap. 15.
ETCHEBEHERE, M. Tumores cartilaginosos malignos: Condrossarcomas. In: Camargo O.P. Clínica Ortopédica. Rio de Janeiro: Med si; 2002. p. 753-759
FELDMAN, F. Cartilaginous tumors and cartilage-forming tumor like conditions of the bonés and soft tissues. In:Diseases of the Skeleton System (Roentgen Diagnosis). Part. 6 – Bone Tumors, New York, Springer-Verlag, 1977,p.177.
FLETCHER, C.D.M., Unni K.K., OMS – Merters F. (Eds.): World Health Organization. Classification of Tumors. Pathology and Genetics of Tumors of Soft Tissue and Bone. IARC Press: Lyon 2002.
GREENSPAN, A. Radiologia ortopédica. Rio de Janeiro: Guanabara; 2001.
HENDERSON, E.D.; Le PAGE, G. A. Apud FELDAMAN, F. Cartilaginius tumors and cartilage forming tumor like conditions of the bone and soft tissues. In: Disease of the Skeletal System (Roentgen Diagnosis). Part. 6 – Bone tumors, New York, Springer Verlag, 1977, p.182.
HUVOS, A.G. Bone tumors Diagnosis, Treatment and Prognosis. Philadelphia, W. B. Saunders Co., 1979, p. 13.
JAFFE, H.L. Tumores y estados tumorales oseos y articulares. México: La Prensa Medica Mexicana; 1966.
JESUS-GARCIA, R. – Reynaldo Jesus-Garcia
LICHTENSTEIN, L. Barcelona: Talleres Gráficos Ibero-Americanos; 1975.
LICHTESTEIN, L. Bone Tumor. 4 Ed St. Louis, C.V. Mosby Co., 1972, cap. 15.
LICHTESTEIN, L.; BERNSTEIN, D. Unusual benign and malignant chondroid tumors of bone. Cancer, 12:1142, 1959.
MARCOVE, R.C. Condrosarcoma: Diagnóstico y tratamiento. In: Clínicas Ortopécias de Norteamérica. Tumores del aparato musculosquelético. Buenos Aires, Panamericana, 1977, cap. 7.
MARCOVE, R.C. et al. Chondrosarcoma of the pélvis and upper end of the femur. Na analisys of factors influencing survival time in113 cases. J. Bone Joint Surg., 54A:61, 1972.
MARCOVE, R.C.; SHOJI, H,; HARLEN, M. Altered carbohidrate metabolism in cartilaginous tumors. Contemp. Surg. 5:53, 1974.
McFARLAND, G.B.Jr.; McKINLEY, L.M.; REED, R.J. Dedifferentiation of low grade chondrosarcomas. Clin. Orthop., 122:157, 1971.
MENENDEZ, L.R. Orthopaedic knowledge update: Actualizaciones en cirugía ortopédica y traumatología. Barcelona: Ars Medica; 2003.
O’NEAL, L.W.; ACKERMAN, L. V. Chondrossarcoma of boné. Cancer, 5:551, 1952.
PRÓSPERO, J.D. Tumores Ósseos. São Paulo, Roca, 2001, cap. II.
ROBBINS. Patologia estrutural e funcional. Rio de Janeiro: Guanabara; 1996.
ROMSDAHL, M.; EVANS, H.L.; AYALA, A.G. Surgical treatment of chondrosarcoma. In: Managment of primary bone and soft tissue tumors. Chicago, Year book med. Publisher Inc., 1977, p. 125.
SCHAJOWICZ, F. Tumores y Lesiones Seudotumorales de Huesos y Articulaciones. Buenos Aires: Editora Médica Panamericana; 1982.
TORNBERG, D.N.; RICE, R.W.; JOHNSTON, A.D. The ultrastructure of chondromyxoid fibroma. Clin. Orthop. Rel. Research, 95:295, 1973.