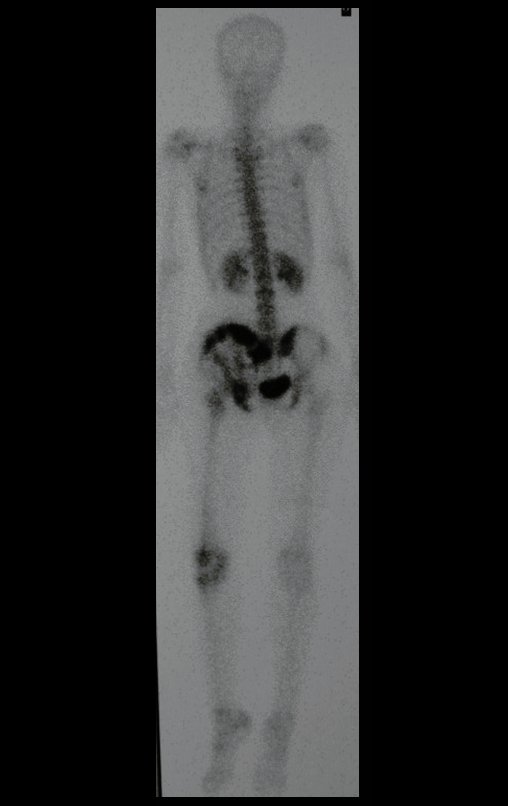
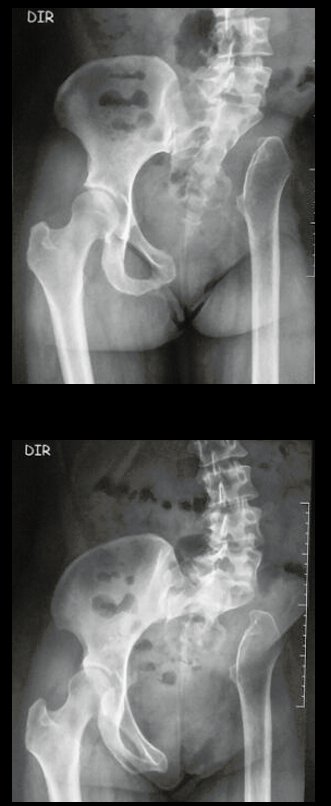
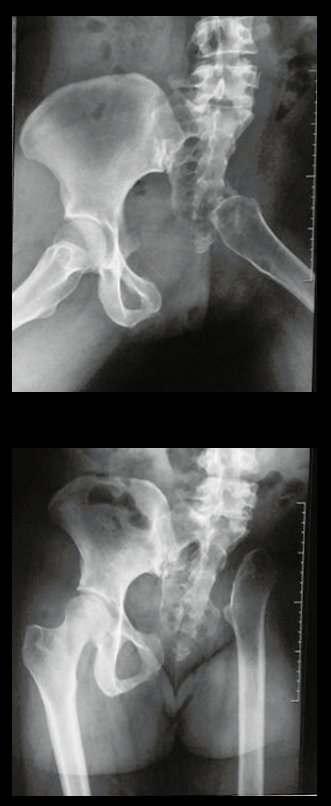
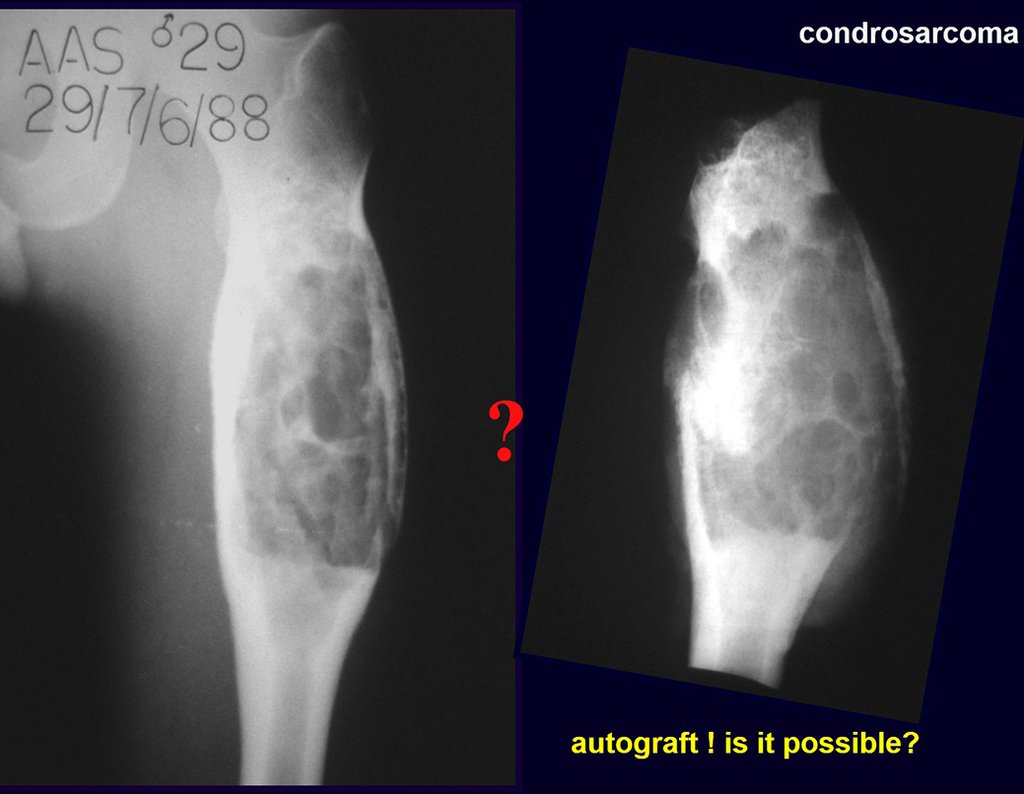
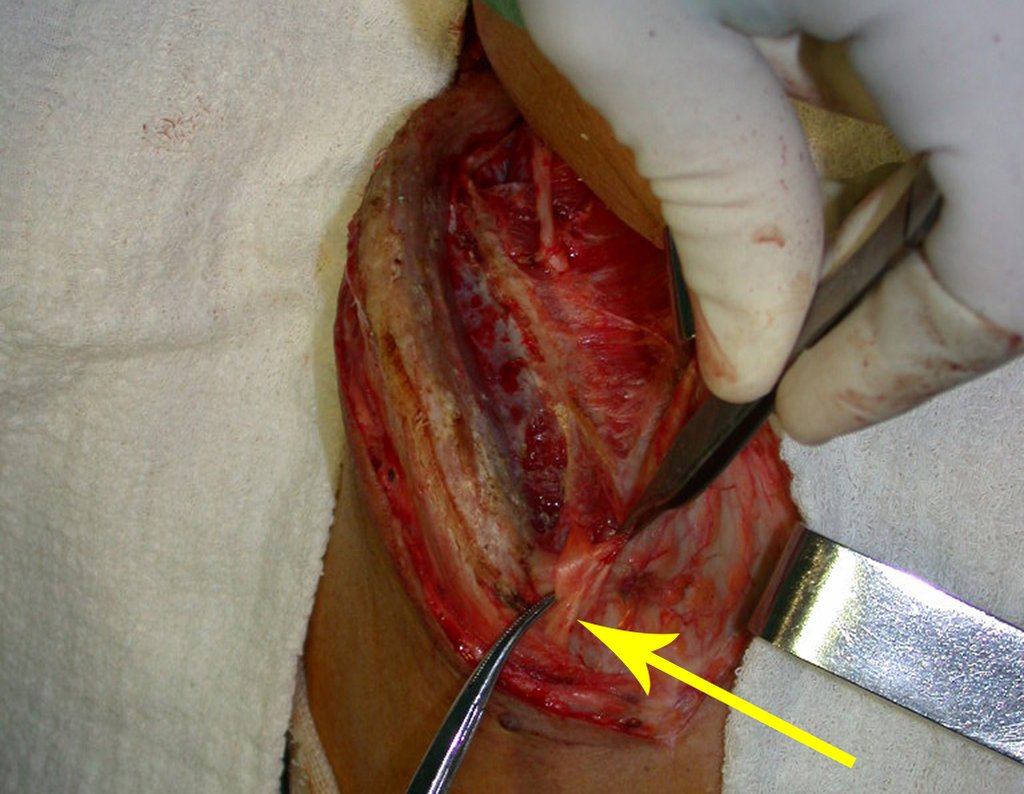
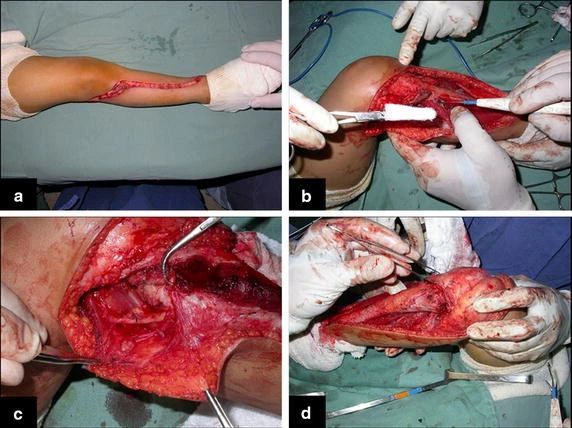
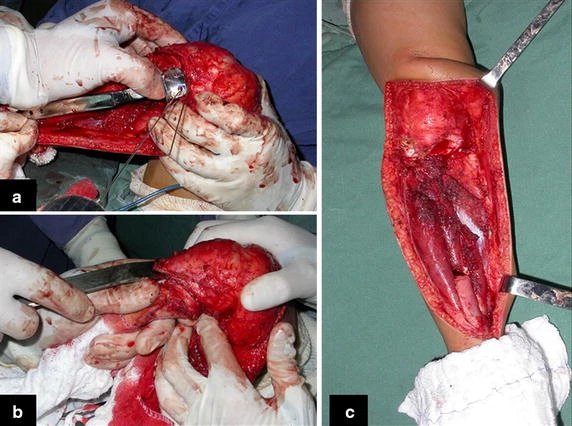
Reconstruction of bone defects resulting from resection of tumors compromising the growth cartilage of the proximal tibia in children represents a challenge to the orthopedist. Due to the low frequency of these lesions, this is a rare situation, in which our technique is for a specific indication: Proximal tíbia tumors resections when the growth line must be removed for oncological reason, but the epiphysis can be preserved, in children with remnant limb growth potential.
Yoshida et al. (2010), has compared multiple reconstruction alternatives at the knee level after tumor resection. They demonstrated that when epiphyseal maintenance is feasible, the vascularized fibular graft or callotasis techniques offers the highest score in the MSTS rating system (Enneking et al. 1993).
Distraction osteogenesis with external fixator eliminates the need of graft and allows padding of the bone defects created by tumor resection through callostasis. It requires, however, long periods with an external fixator device, which increases the risk of infection in patients potentially immunosuppressed by adjuvant oncological treatment (Kapukaya et al. 2000).
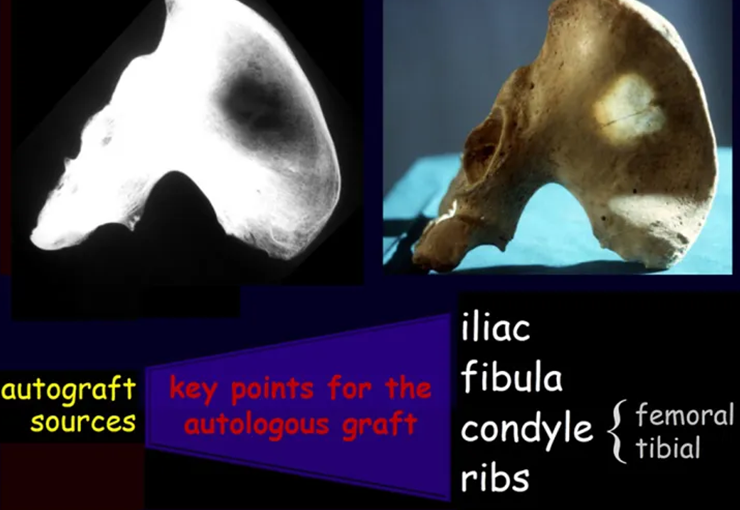
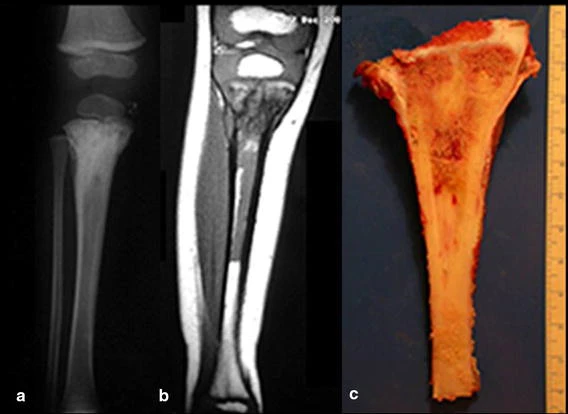
Bone grafts have been used for reconstruction of proximal tibial resections, especially in cases where the tibial epiphysis can be preserved (Honoki et al. 2008), which occurs in up to 20 % of cases (Norton et al. 1991; Simon and Bos 1980). When the graft is not vascularized, the technique presents high rates of fracture or non-union, and it does not prevent further limb discrepancy (Muscolo et al. 2004). Weitao et al. (2012) reported the use of allograft in the proximal tibia in 5 patients after tumor resection preserving the epiphysis. Delayed bone healing in Allograft-host junction were seen in all cases.
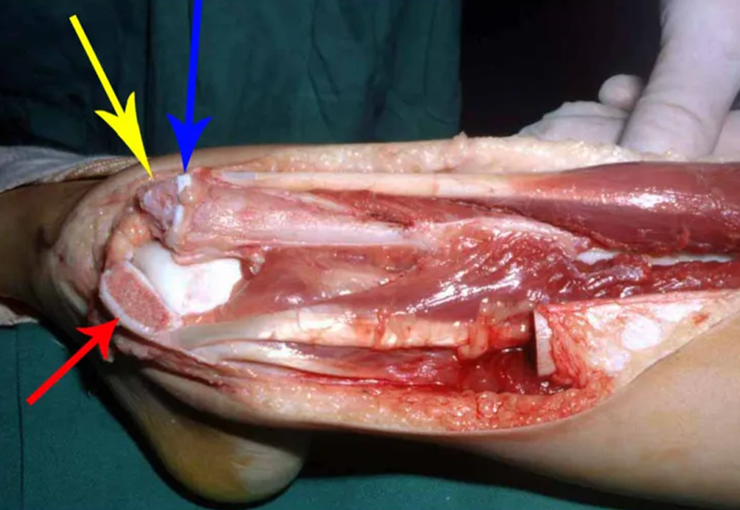
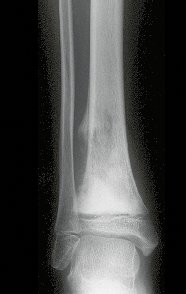
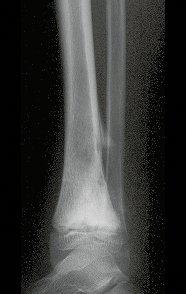
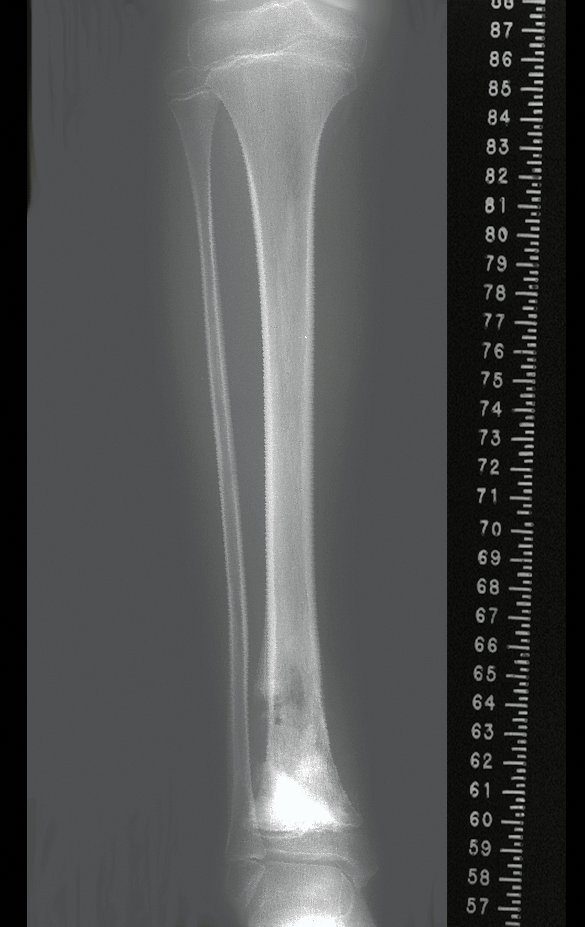
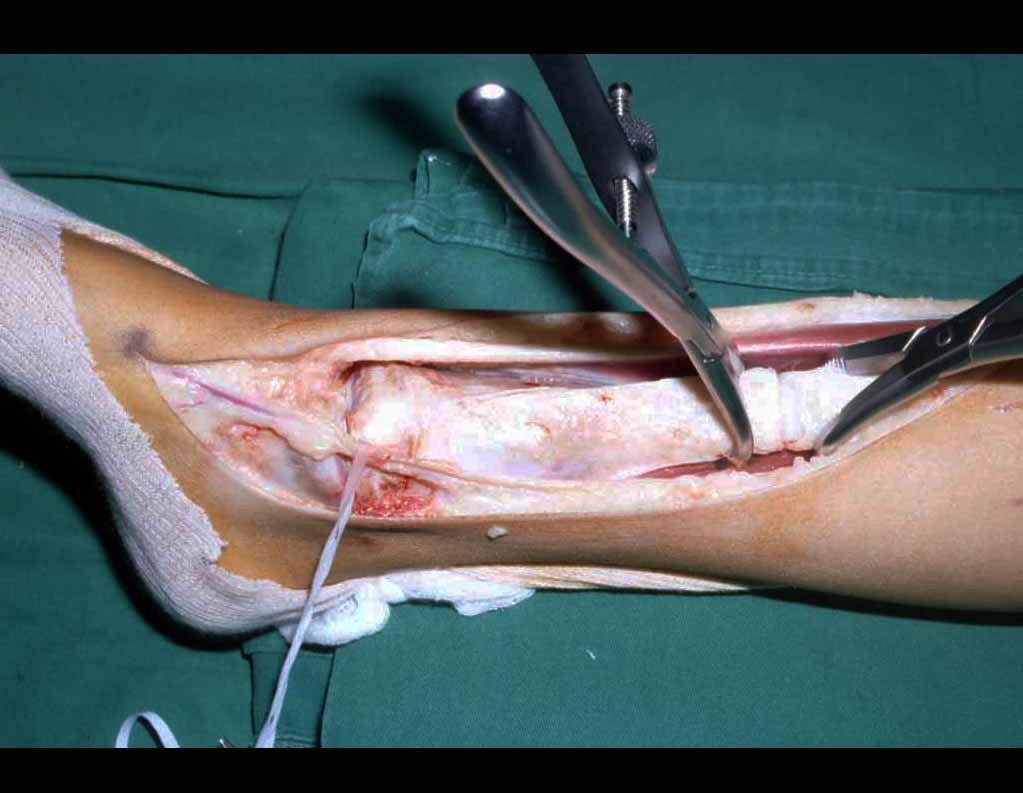
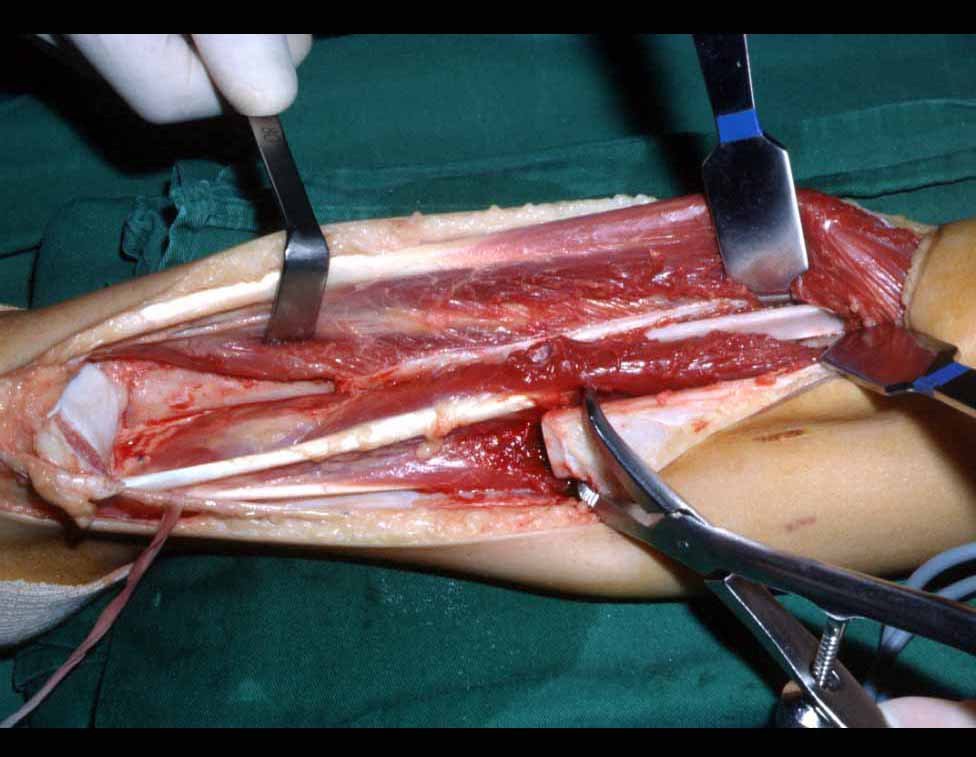
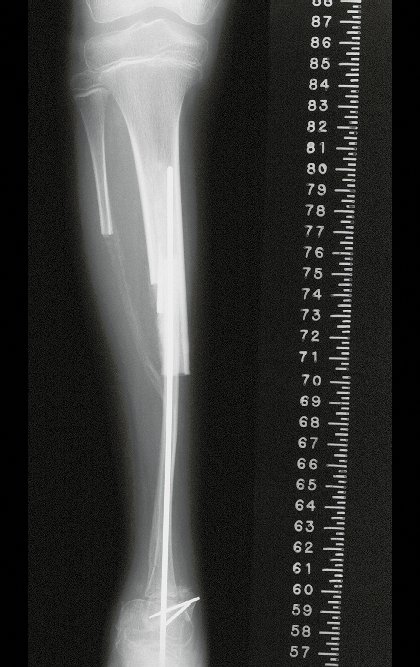
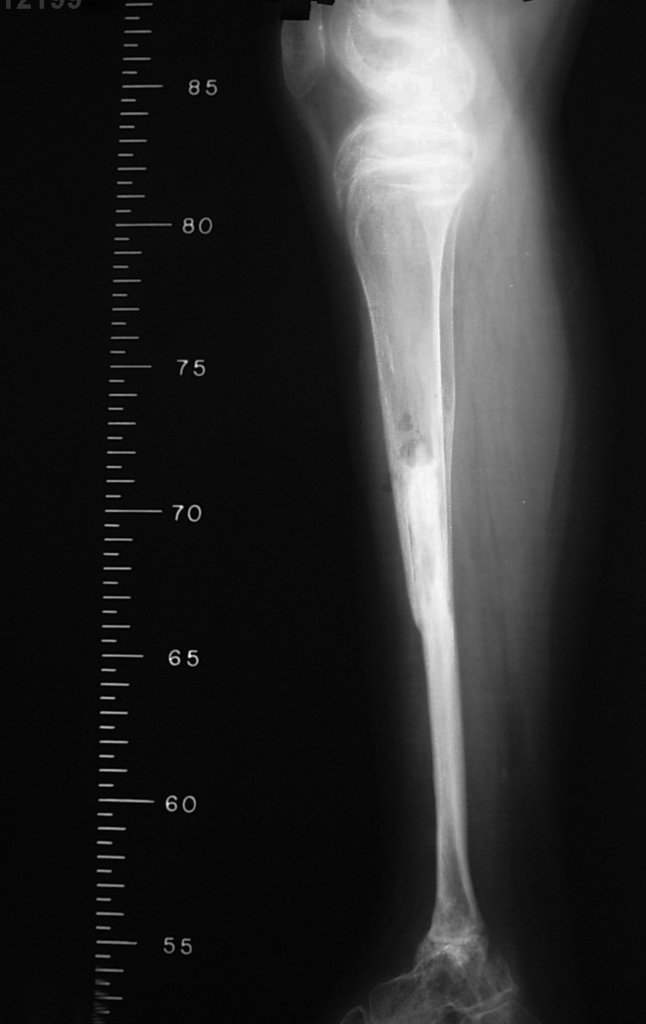
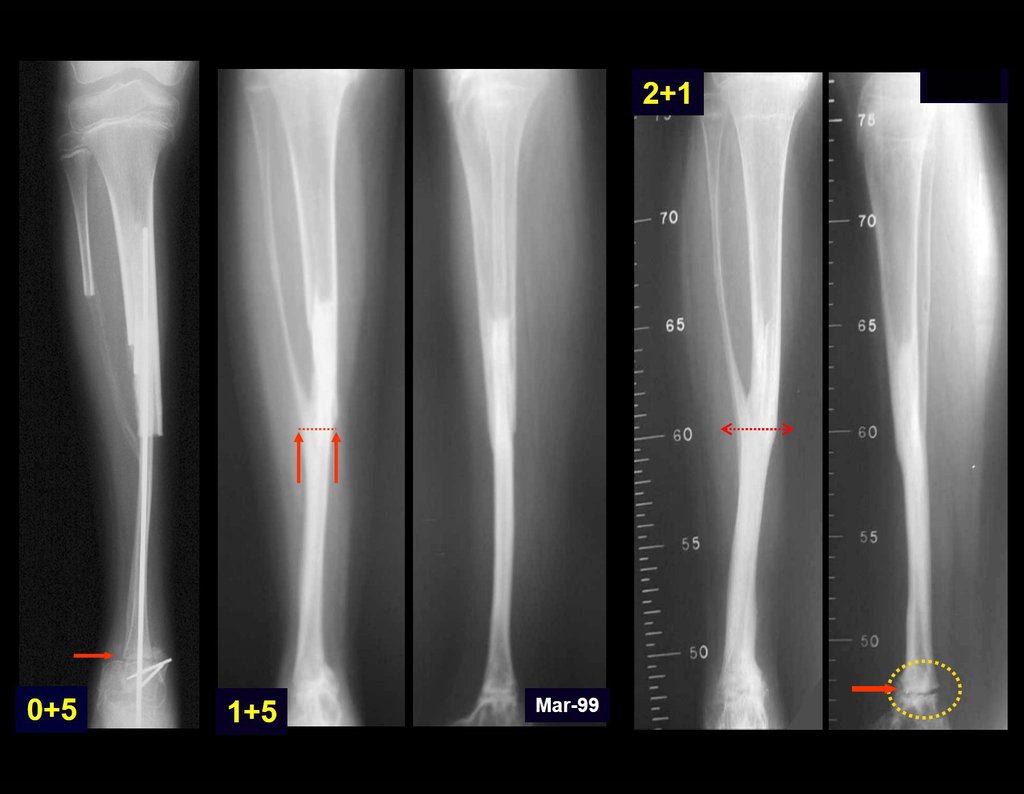
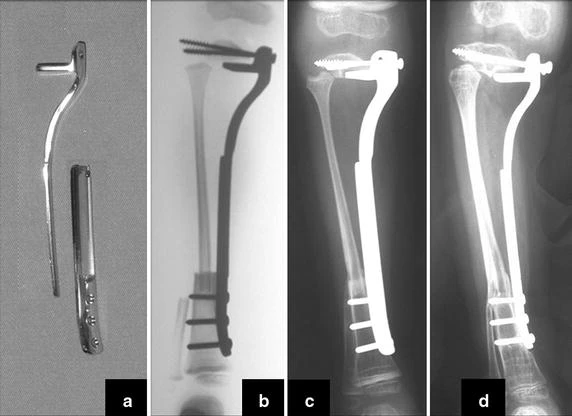
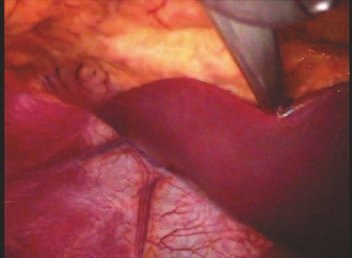
Transposition of the fibula shows advantages over the allograft in the bone healing process. Since it is a bone-muscle flap with natural vascularization, bone turnover is preserved and actively participates in the process of bone healing, while the growth potential of the physis is maintained. As seen in Figs. 7 and 9, the fibula undergoes progressive hypertrophy and strengthening, in contrast to allograft, which may fail even years after integration (Date et al. 1996; Hriscu et al. 2006).
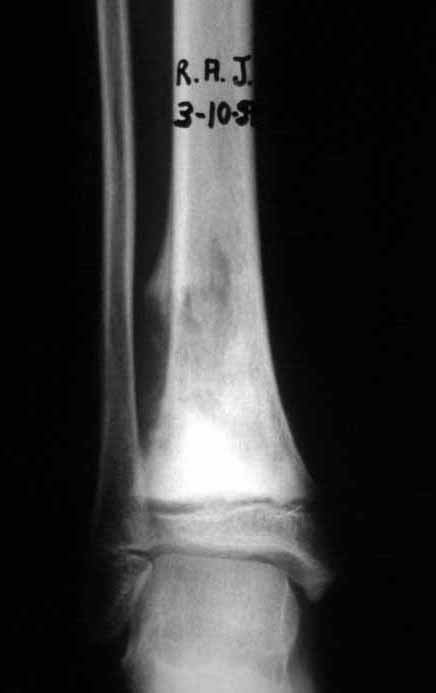
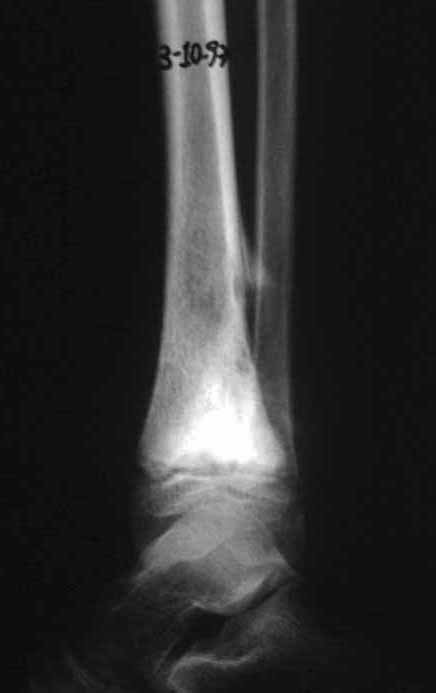
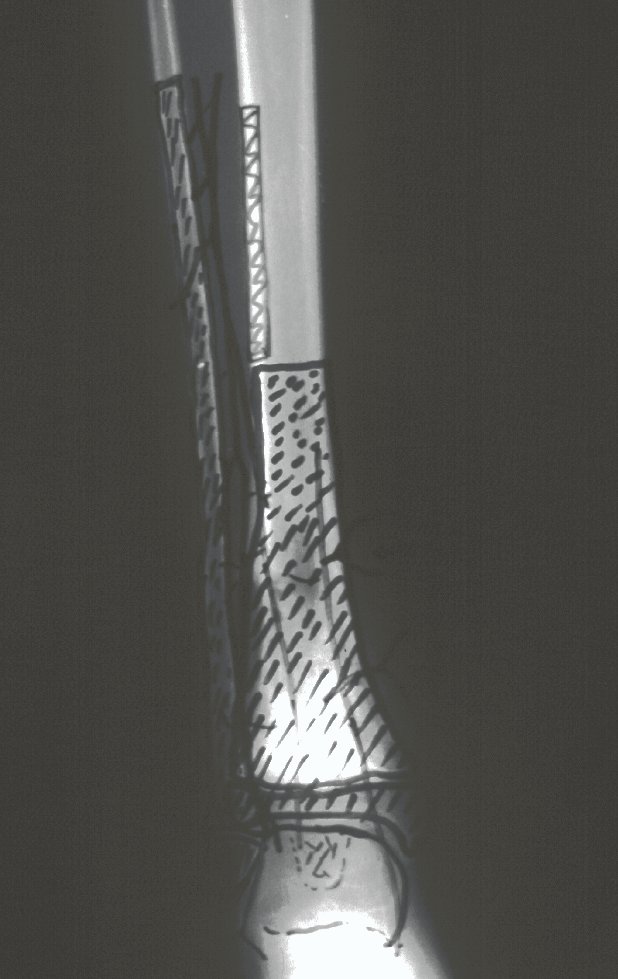
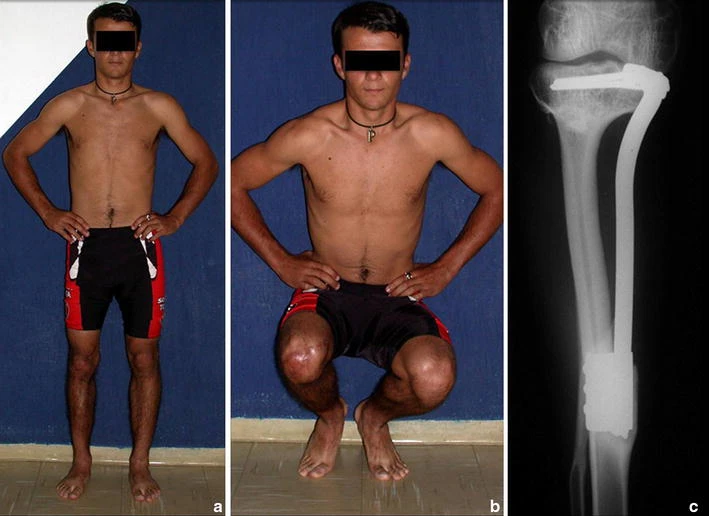
The transfer of the fibula with its growth plate as a reconstruction method requires long term rehabilitation and late resuming of loadbearing ambulation, until bone healing of the tibia and the transposed fibula occurs. Hypertrophy of the fibula, which was reported in our two cases, evidences that the bone has achieved enough resistance required for loadbearing. Since this is a biological reconstruction method, once consolidation and hypertrophy occur, it can be considered a definitive solution, carried out with a single surgical procedure. The preservation of the proximal epiphysis of the tibia while maintaining the articular surface of the knee by trans epiphyseal osteotomy represents a mechanical advantage. It is also a necessary condition to use a fibular transposition. The maintenance of longitudinal growth of the transposed fibular segment, as documented in our two patients, is of major importance, since it can prevent or minimize the final discrepancy of the lower limbs length after growth.
Capanna et al. (2007) presented a original solution for long bones intercalary defects reconstruction associating massive allograft with vascularized fibular autograft. Good results were presented in 57 patients with proximal tibia reconstruction, nevertheless this technique does not address final limb discrepancy. The authors developed a good alternative to reconstructions of bone defect of the proximal tibia, but require microsurgery technique, which increases the cost of the procedure and the availability of bone allograft compatible in size with the patient, which is not widely available in some countries.
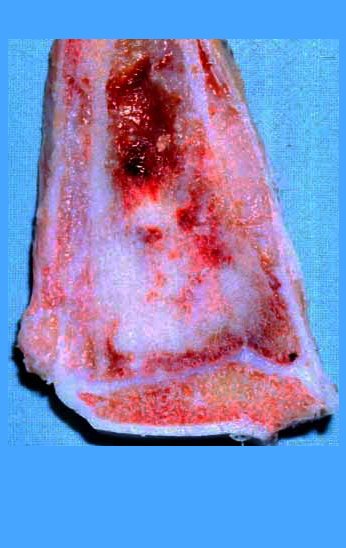
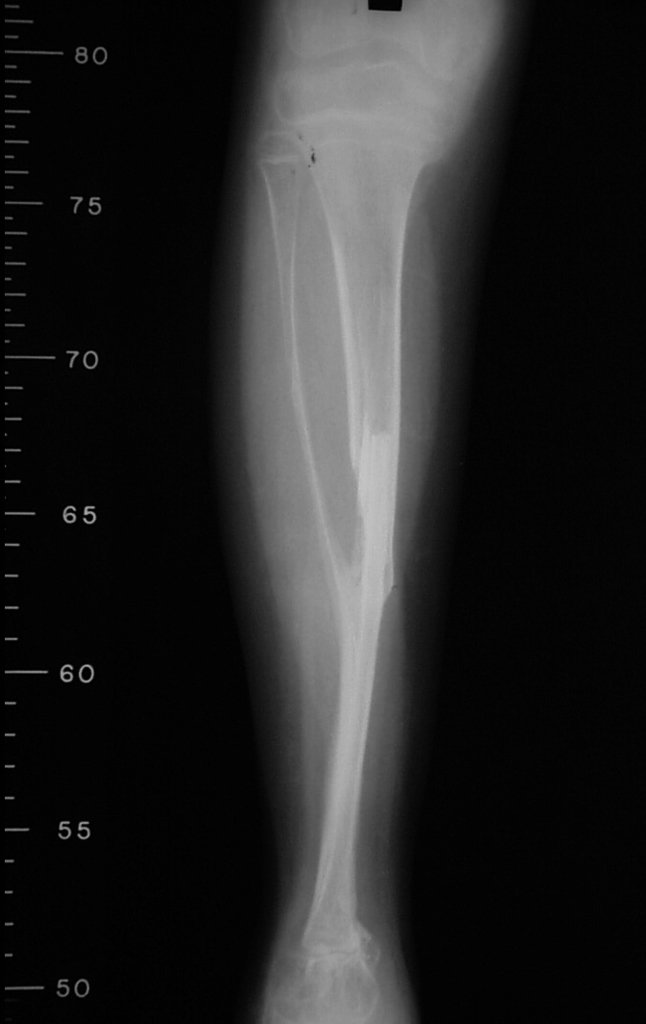
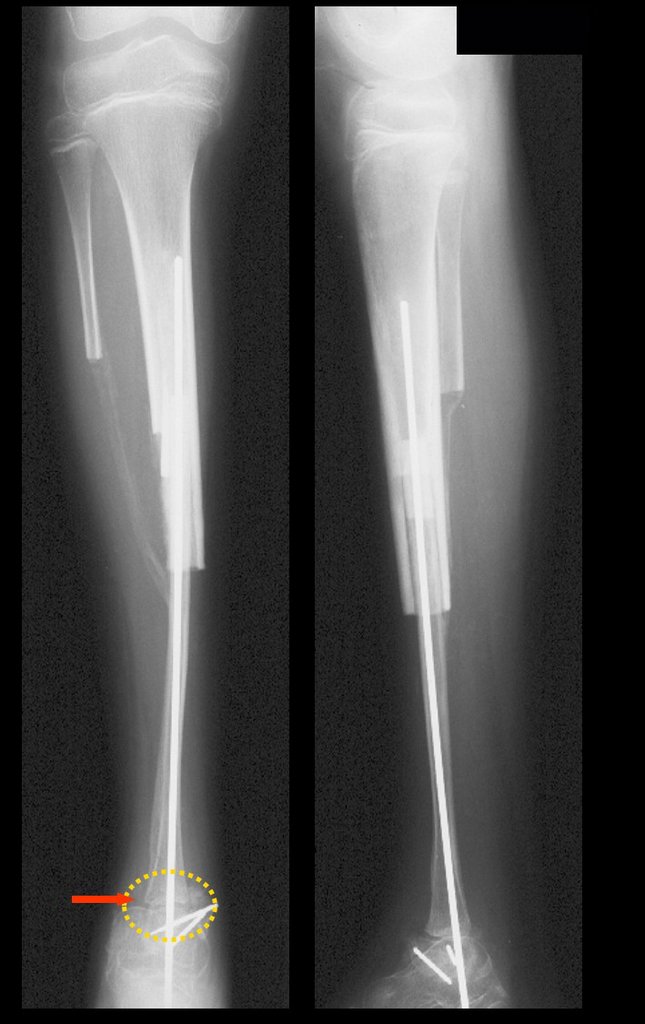
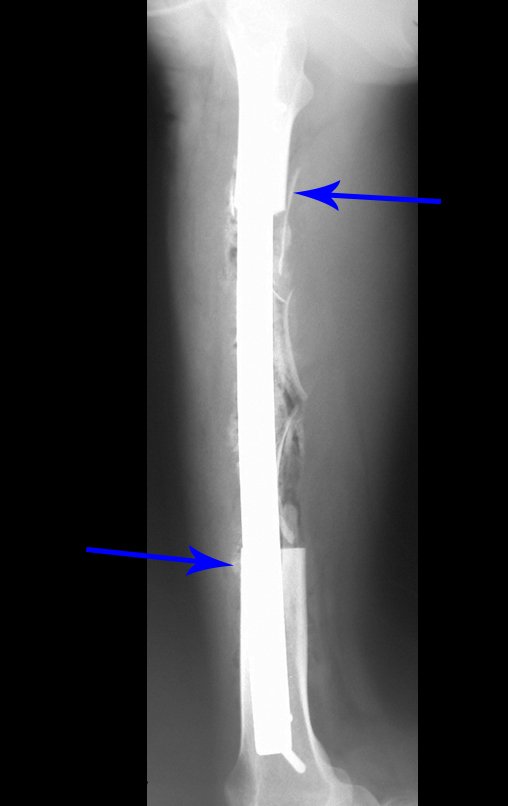
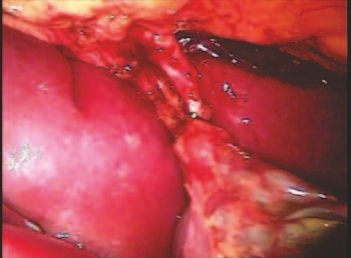
Our technique has the advantage to waive any method of vascular reconstruction of the fibular segment, since vessels are preserved. Fibular hypertrophy and longitudinal growth of the transplanted bone segments were observed in the two reported cases. Distal bone healing was succeeded in both cases. Proximally, there was a non union in the second case, due to the non resection of the proximal fibular articular cartilage which is essential for bone healing. This patient was schedule to a second procedure to resect the articular cartilage of the proximal fibula, but he had a clinical complication, from chemotherapy, which led to death. During the first 8 months after surgery, however, growth of at least 0.3 cm of the transposed fibula was documented, suggesting preservation of the growth potential (Fig. 9).
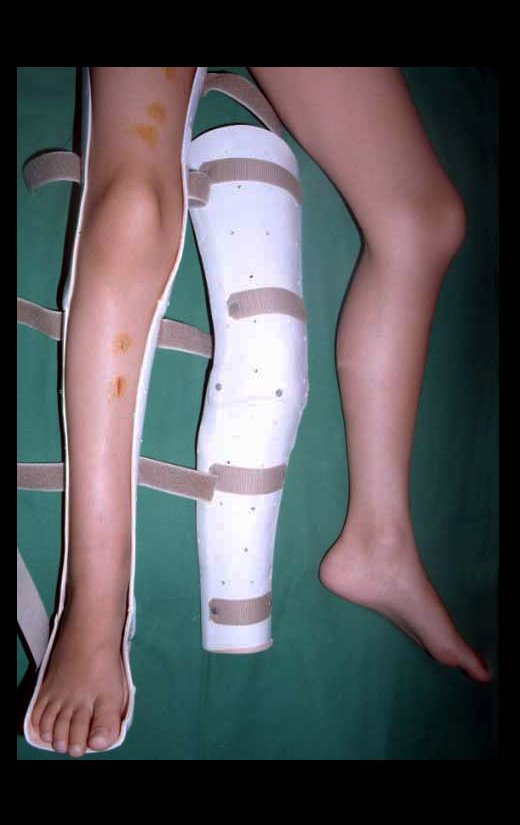
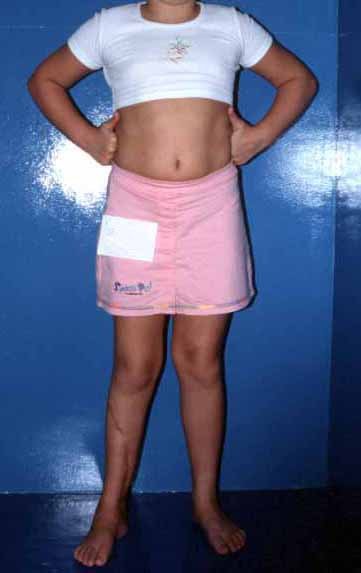
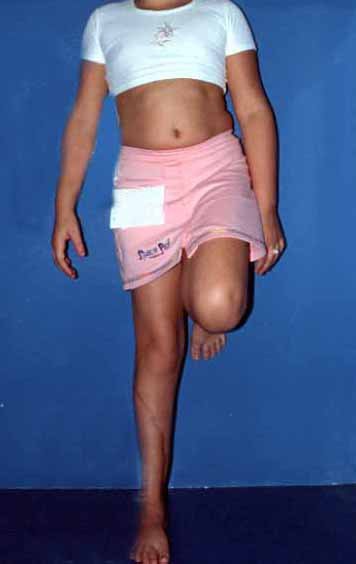
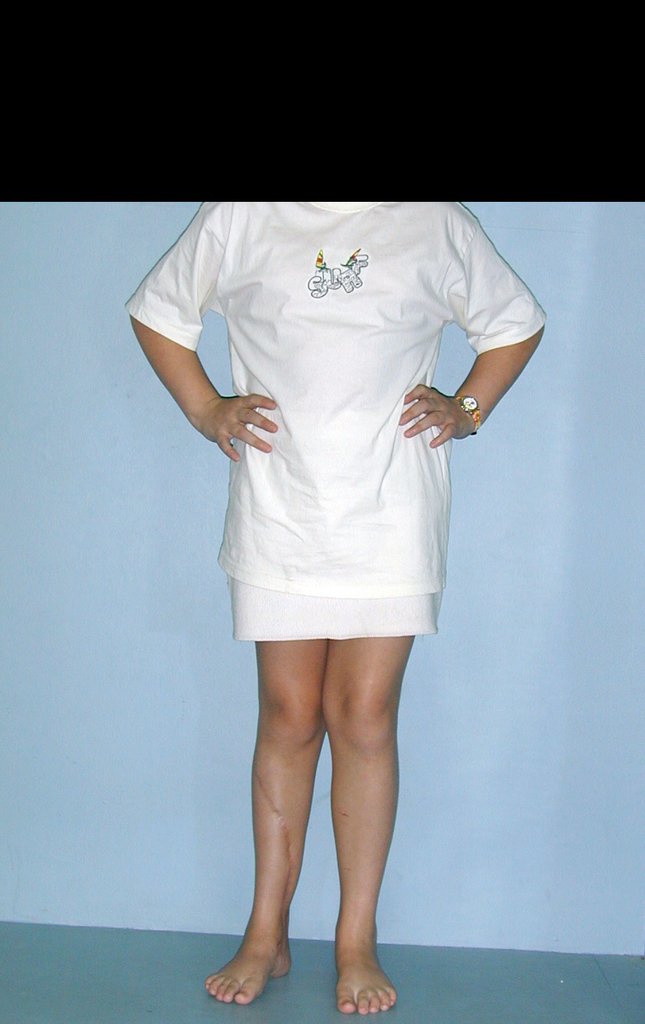
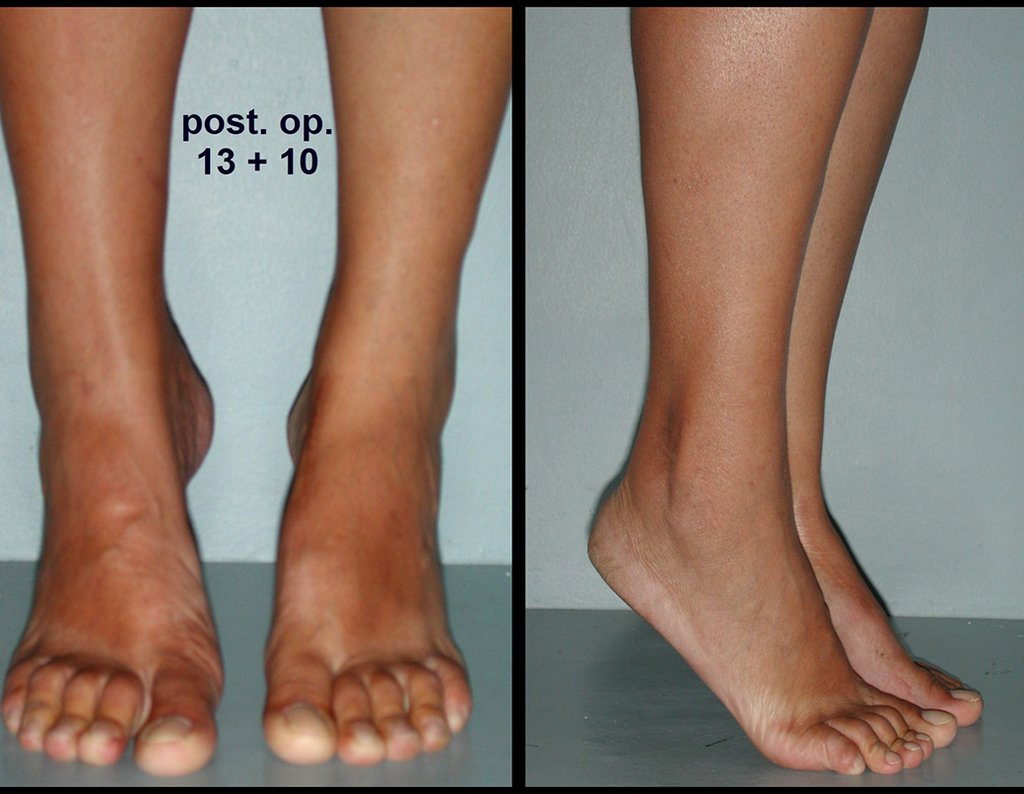
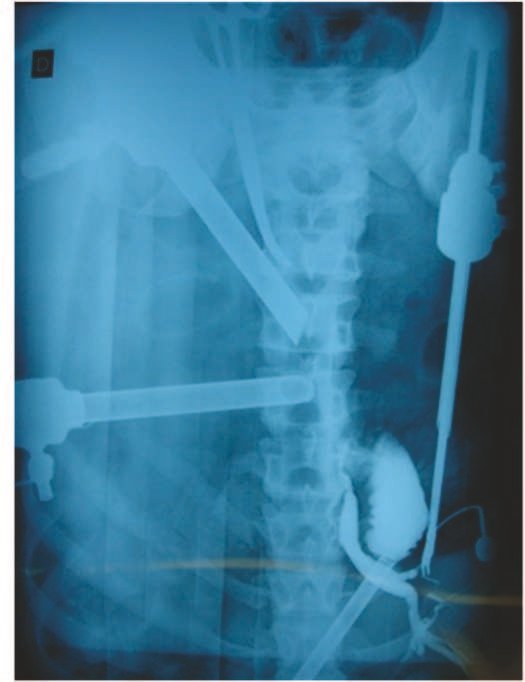
The first patient developed a valgus deformity of the right knee in the immediate postoperative period, which was corrected within the first post-operative year, after bone growth and correction of slope of the screws (Fig. 7). Long term resolution of the tibial reconstruction defect was observed in this patient, as documented by the equal length and normal function of lower limbs in adulthood. In spite of the restricted indication, we believe that our technique can positively affect the long term outcome of young patients undergoing reconstruction of proximal tibial defects.
Reconstruction of the proximal tibia with a megaprosthesis represents a therapeutic option when epiphysis must be resected. It allows limb preservation and early ambulation but High rates of complications have been reported such as infection, aseptic loosening, mechanical failure, and limitation to physical activities (Saghieg et al. 2010; Gosheger et al. 2006; Campanacci et al. 2010; Fang et al. 2013). This method requires multiple surgical procedures for revisions, reaching 42 % implant failures in 10 years follow up (Jeys et al. 2008). When epiphyseal maintenance is feasible, megaprosthesis has fewer indications. When early ambulation is priority for low expectation of life patients.