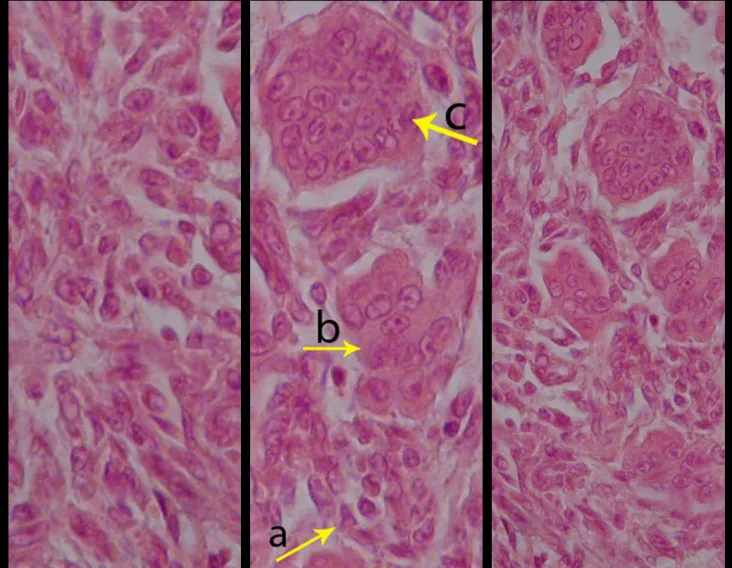
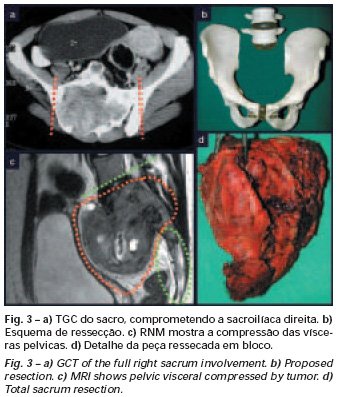
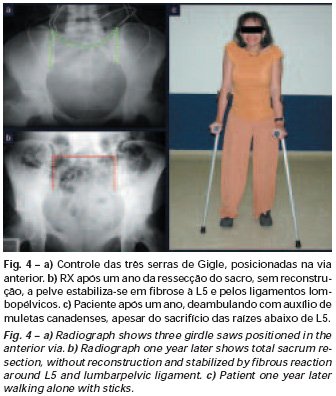
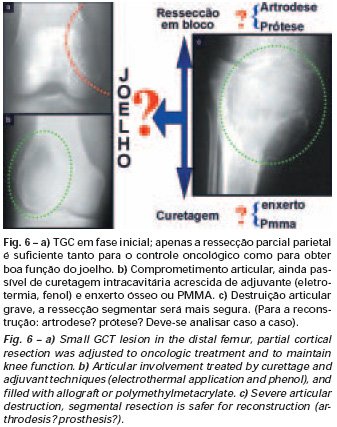
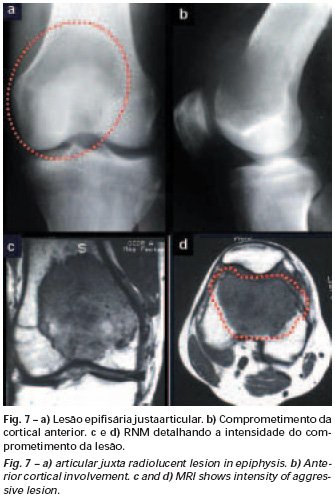
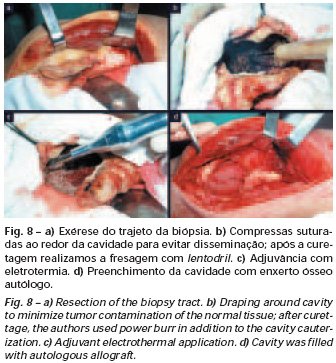
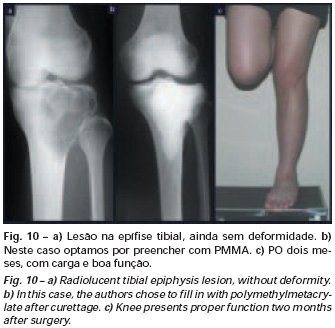
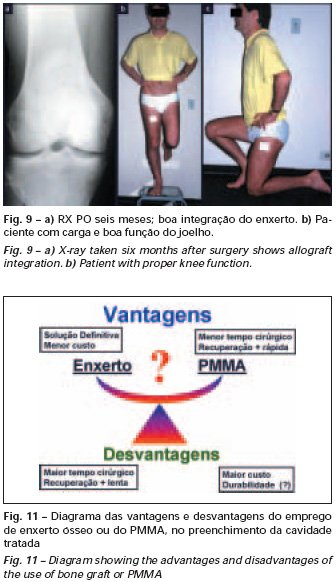
Bone grafting is a biological and definitive solution; however, it makes it difficult to visualize possible early recurrence, which can be confused with the physiological reabsorption of the graft integration process, in addition to requiring around six months, on average, for full load. The non-autologous homologous graft has a longer integration period and is not always available, but, on the other hand, it shortens surgical time. The autologous graft has the advantage of immunocompatibility and faster integration, but it prolongs surgical time. Due to the risk of malignant transformation, radiotherapy can only be considered as a treatment option for giant cell tumors located in structures that are difficult to access surgically. Therefore, especially for the knee region, the orthopedist familiar with the treatment of oncological lesions must evaluate the clinical and radiographic aspects, the degree of joint destruction, the patient’s profession, in short, all pertinent factors, in order to make the best indication. therapy(37). Complications inherent to this tumor are recurrences, sinking of the articular surface, leading to varus, valgus, antecurvatum or retrocurvatum deviations. Exceptionally, lung metastases or malignancy may occur(43-46).REFERENCES1. Cooper A., Travers B.: Surgical Essays. London, Cox and Son, 1818. 2. Paget J.: Lectures on Surgical Pathology. London, Longmans, 1853. 3. Nelaton E.: D’une Nouvelle Espece de Tumeurs à Mieloplaxes. Paris, Adrien Delahaye, 1860. 4. Gross SW: Sarcoma of the long bones: based upon a study of one hundred and sixty five cases. Am J Med Sci 78: 17-19, 1879. 5. Bloodgood JC: Benign giant cell tumor of bone: its diagnosis and conservative treatment. Am J Surg 37:105-106, 1923. 6. Geschikter DF, Copeland NM: Tumors of Bone. Philadelphia, JB Lippincott, 1949. 7. Willis GE: The pathology of osteoclastoma or giant cell tumors of bone. J Bone Joint Surg [Br] 31: 236-238, 1949. 8. Jaffe HL, Lichtenstein L., Portis R., RB: Giant cell tumor of bone. Pathological appearance, grading, supposed variants and treatment. Arch Pathol 30: 993-995, 1940. 9. Sherman M.: “Giant cell tumor of bone” in Tumors of bone and soft tissue. Chicago, Year Book Medical Publishers, 1965. 10. Schajowics F.: Giant cell tumors of bone (osteoclastoma): a pathological and histochemical study. J Bone Joint Surg [Am] 43: 1-3, 1961. 11. Hanaoka H., Friedman NB, Mack RP: Ultrastructure and histogenesis of giant cell tumor of bone. Cancer 25: 1408-1423, 1970. 12. Wilson Jr. PD: A clinical study of the biochemical behavioral defects. Clin Orthop 87: 81-109, 1972. 13. Ackerman M., Berg NO, Person BM: Fine needle aspiration biopsy in the evaluation of tumor-like lesions of bone. Acta Orthop Scand 47: 129-136, 1976. 14. Eideken J., Hodes PJ: Roentgen Diagnosis of Diseases of Bone. Baltimoremore, Williams and Wilkins, pp 6588-6593, 1967. 15. Chaix C., Trifaud A.:Evolution and treatment of giant cell tumors of bone. Rev Chir Orthop 61: 429-434, 1975. 16. Derqui JC: Diagnosis of Giganto-cellular Osteopathies in Childhood. Buenos Ayres, Macchi HMS, 1962. 17. Lichtenstein L: Current status of problems in diagnosis and treatment. J Bone Joint Surg [Am] 33: 143-152, 1951. 18. Tornberg DN, Dick HM, Johnston AD: Multicentric giant cell tumor in the long bones. A case report. J Bone Joint Surg [Am] 57: 420-422, 1975. 19. Aegerter E., Kirkpatrick JA: Orthopedic Diseases. Philadelphia, WB Saunders, 1975. 20. Dahlin DC, Cupps RE, Johnson Jr. EW: Giant cell tumor: a study of 195 cases. Cancer 25: 1061-1070, 1970. 21. Goldenberg RR, Campbell CJ, Bonfiglio M.: Giant cell tumor of bone. An analysis of 218 cases. J Bone Joint Surg [Am] 52: 619-664, 1970. 22. Larsson SE, Lorentzon R., Boquist L.: Giant cell tumor of bone. A demographic, clinical and histopathological study recorded in the Swedish Cancer Registry. J Bone Joint Surg [Am] 57: 167-173, 1975. 23. Lichtenstein L.: Bone Tumors. St. Louis, CV Mosby, 1972. 24. McGrath PJ: Giant cell tumor of bone. An analysis of fifty two cases. J Bone Joint Surg [Br] 54: 216-227, 1972. 25. Marcove RC, Weiss LD, Vaghaiwalla MR: Cryo surgery in the treatment of giant cell tumors of bone. A report of 52 consecutive cases. Cancer 41: 957-964, 1978. 26. Rodrigues JS: Giant cell tumors of bone [Thesis]. Recife, Pernambuco, Faculty of Medicine of the Federal University of Pernambuco, 1972. 27. Sissons HA: “Bone tumors” in Wright P., Symmers W. St. C.: Systemic pathology. London, Longmans, Green and Co., 1966. 28. Huvos AG: Bone Tumors Diagnosis, Treatment and Prognosis. Philadelphia, WB Saunders, vol. 17, pp 429-467, 1991. 29. Hutter RVP, Worcester Jr. JN, Francis KC: Benign and malignant giant cell tumors of bone. A clinicopathological analysis of the natural history of the disease. Cancer 15: 663-672, 1962. 30. Levine HA, Eurile F.: Giant cell tumor of patellar tendon coincident with Paget’s disease. J Bone Joint Surg [Am] 53: 335-341, 1971. 31. Jaffe HL: Tumors and tumorous conditions of the bones and joints. Philadelphia, Lea & Febiger, 1958. 32. Cameron GW: Giant cell tumor of the patella. J Bone Joint Surg [Am] 37: 184-187, 1955. 33. Baptista PPR: Treatment of giant cell tumors by curettage, electrothermal cauterization, drill regularization and autologous bone graft. Rev Bras Ortop 30: 819-827, 1995. 34. Ottolenghi CE: Massive osteo and osteoarticular bone graft. Technique and results of 62 cases. Clin Orthop 87: 156-164, 1972. 35. Kambin P.: Giant cell tumor of thoracic spine with pathological fracture paraparesis: a method of stabilization. J Bone Joint Surg [Am] 48: 774-779, 1966. 36. Parrish FF: Treatment of bone tumors by total excision and replacement with massive autologous and homologous grafts. J Bone Joint Surg [Am] 48: 968-990, 1966. 37. Baptista PPR:Treatment of giant cell tumor of the distal end of the femur and proximal end of the tibia – Curettage, electrothermal cauterization and autologous bone graft [Thesis]. São Paulo: Faculdade de Ciências Médicas da Santa Casa de São Paulo, 1994. 38. Murphy WR, Ackerman L.: Benign and malignant giant cell tumor of bone. Cancer 9: 317-324, 1956. 39. Camargo FP: Segmental resection in bone tumors and surgical reconstitution of the skeleton [Thesis]. São Paulo: Faculty of Medicine of the University of São Paulo, 1968. 40. Forrest M.: The pathology of giant cell tumors of bone. Rev Chir Ortho 61: 359-366, 1975. 41. Barnes R.: Giant cell tumor of bone. J Bone Joint Surg [Br] 54: 213-215, 1972. 42. Campbell CJ, Akbarnia BA: Giant cell tumor of the radius treated by massive resection and tibial bone graft. J Bone Joint Surg [Am] 57: 982- 986, 1975. 43. Frangakis EK: Soft tissue spread of giant cell tumor. J Bone Joint Surg [Am] 52: 994-998, 1971. 44. Caballos RL: The mechanism of metastasis in the so-called “benign giant cell tumor of bone”. Hum Pathol 12: 762-768, 1981. 45. Schajowics F.: About the degeneration and malignant variety of giant cell tumors. Rev Orthop Traum 10: 349-355, 1941. 46. Riley LD, Hartmann W.: Soft tissue recurrence of giant cell tumor of bone after irradiation and excision. J Bone Joint Surg [Am] 49: 365-368, 1967.